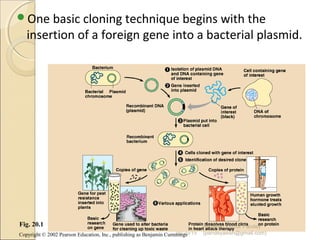
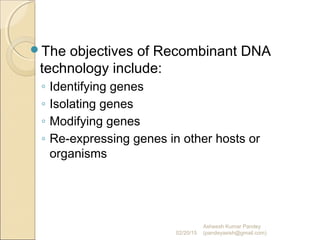
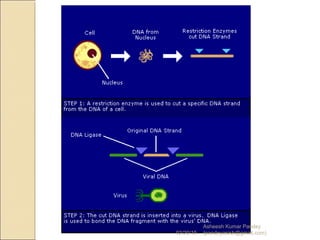
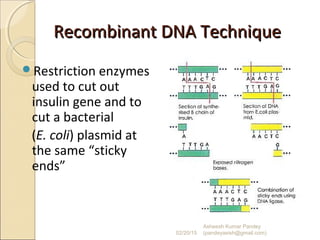
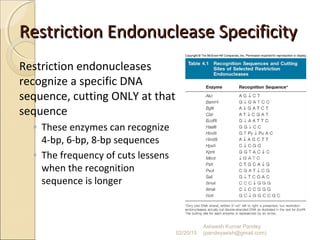
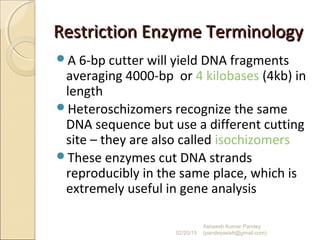
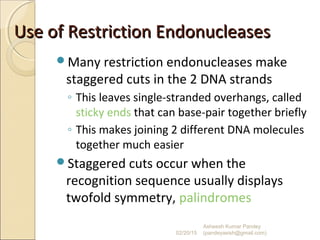
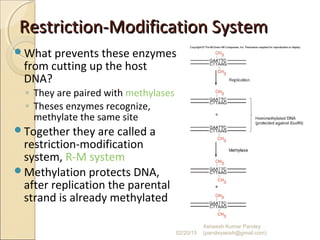
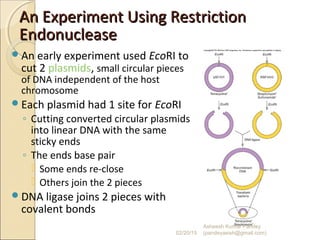
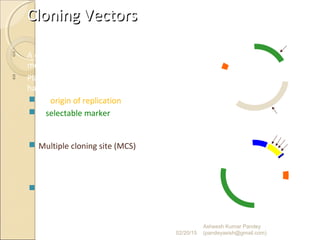
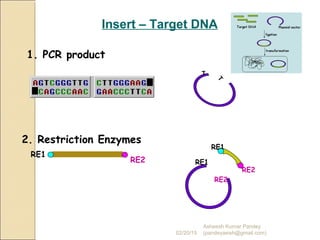
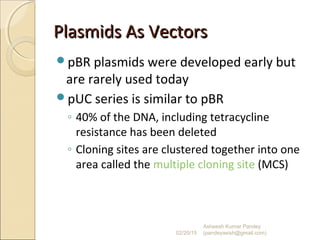
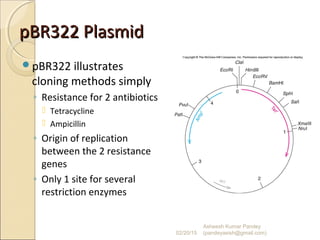
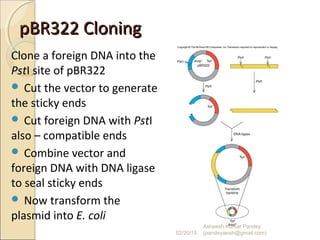
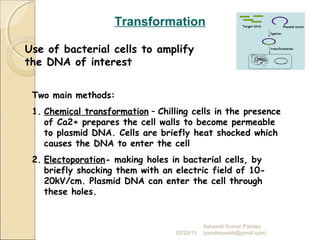
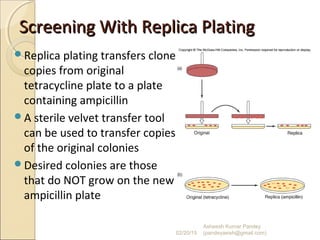
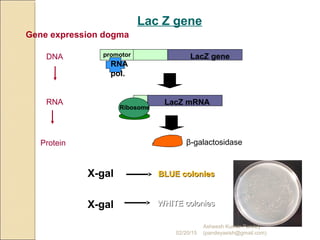
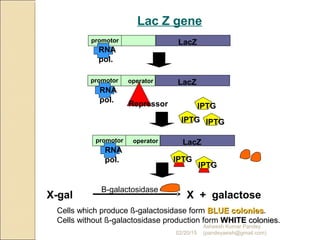
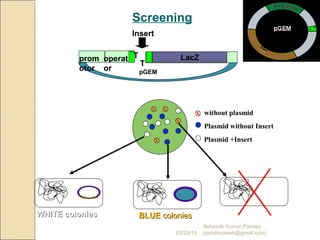
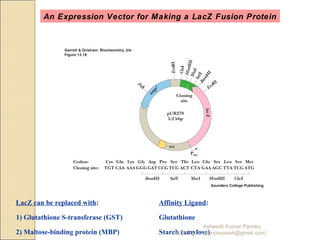
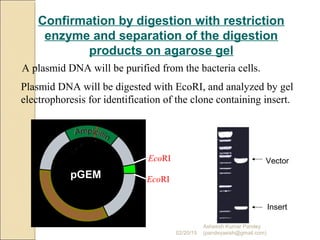
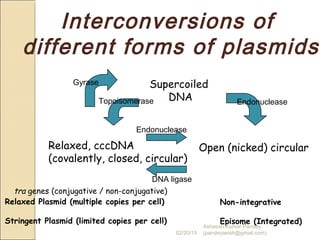
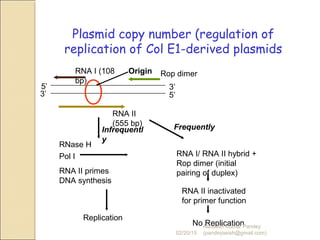
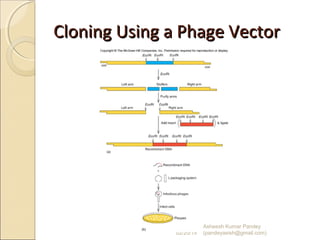
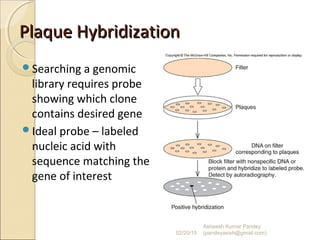
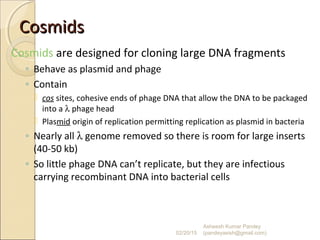
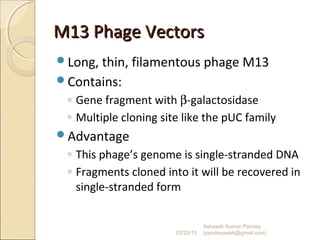
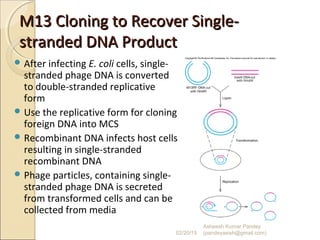
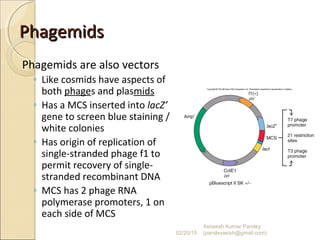
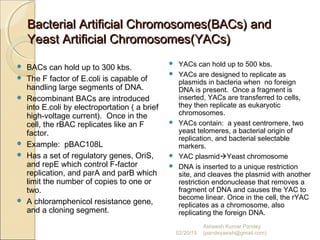
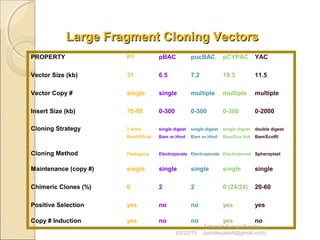
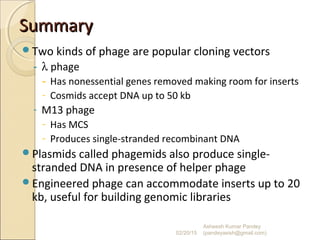
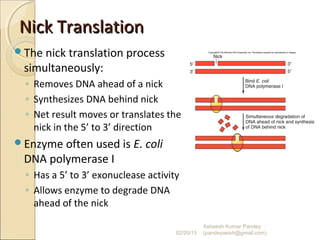
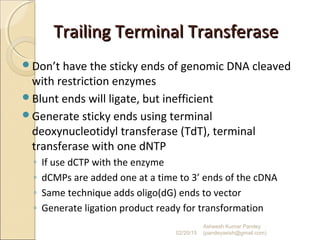
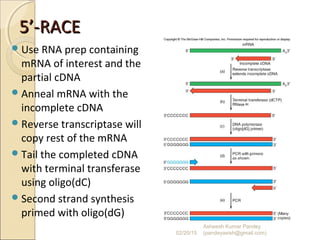
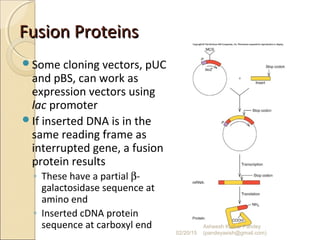
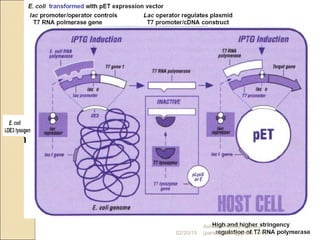
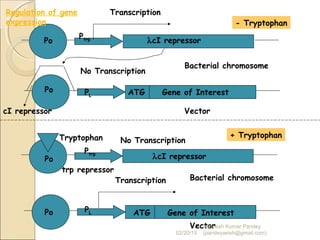
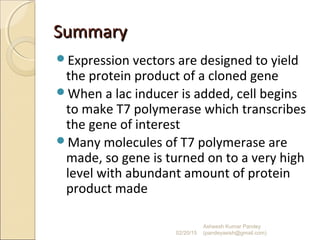
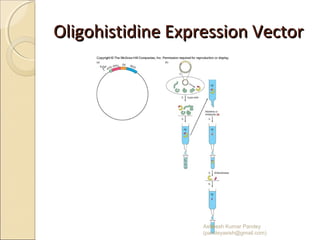
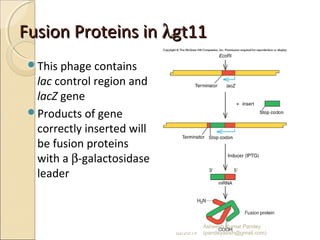
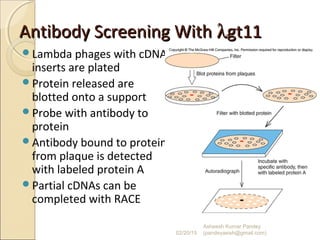
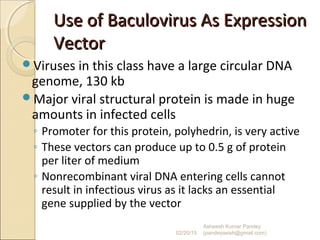
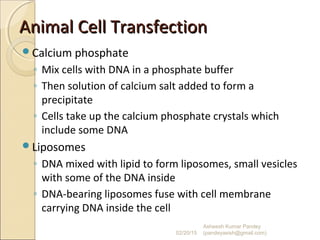
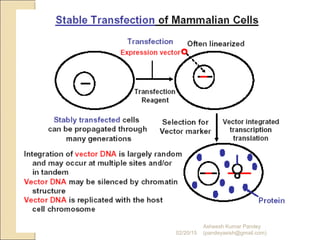
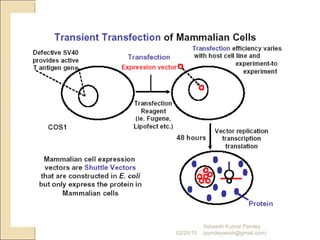
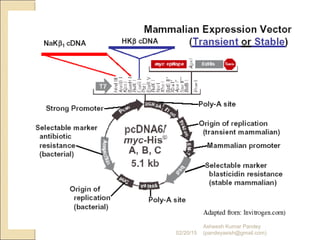
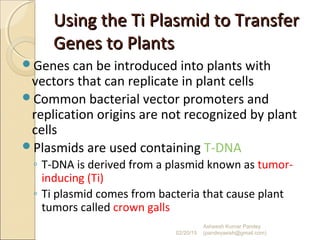
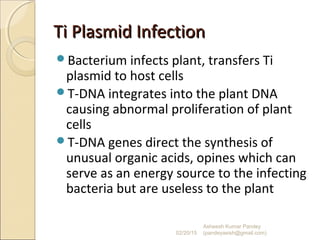
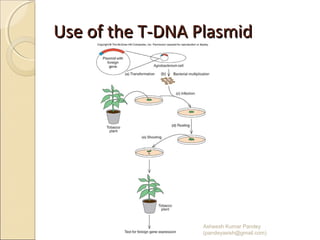
The document discusses cloning and recombinant DNA technology. It defines cloning as making identical copies of a molecule such as a gene. Recombinant DNA technology allows genes to be isolated, modified, and expressed in new hosts. One technique is inserting a foreign gene into a bacterial plasmid, which then replicates and produces multiple copies of the gene. Restriction enzymes and ligases are used to cut and paste DNA fragments for cloning.