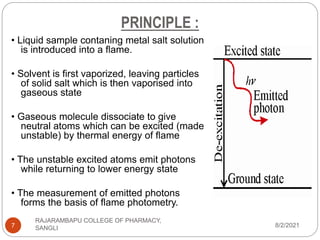
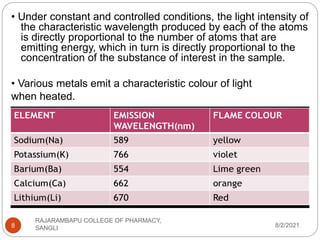
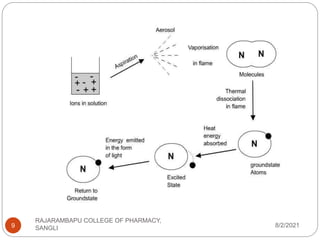
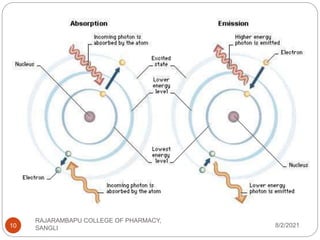
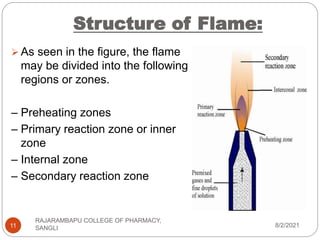
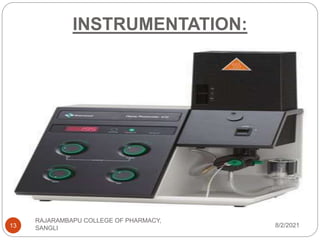
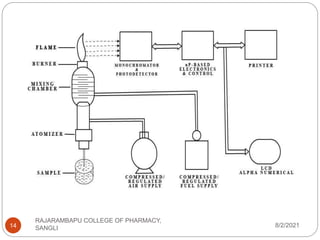
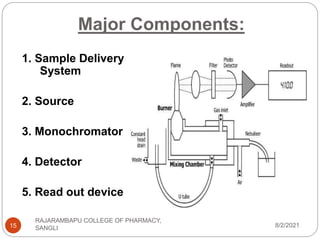
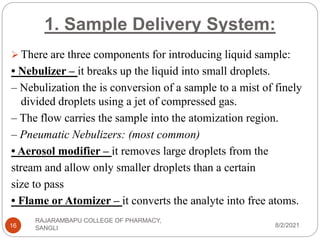
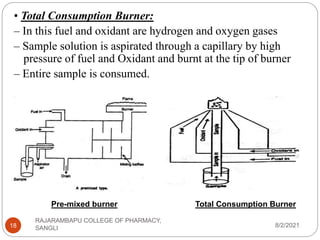
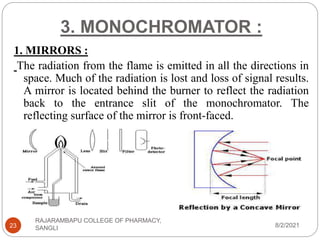
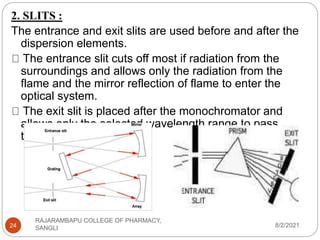
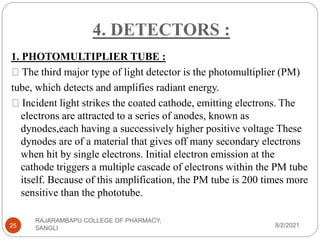
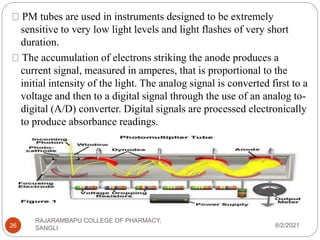
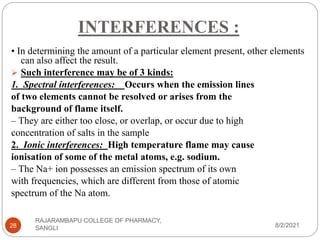
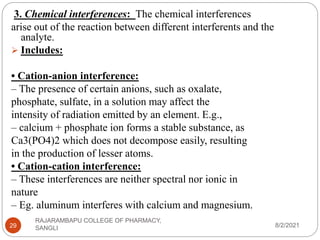
Flame photometry is a technique used to determine the concentration of certain metal ions in a sample by measuring the intensity of light emitted from their atoms when excited in a flame. The sample is nebulized and introduced into a flame, where the metal atoms are excited and emit light of characteristic wavelengths. This emitted light is separated into its component wavelengths by a monochromator and measured with a detector, allowing quantification of metal ion concentrations in the original sample. Potential sources of interference include overlap of emission lines from different elements and effects of high ion concentrations on atom excitation.