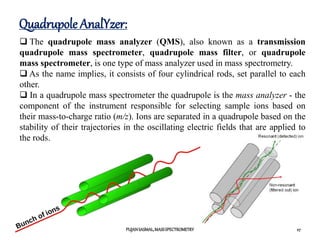
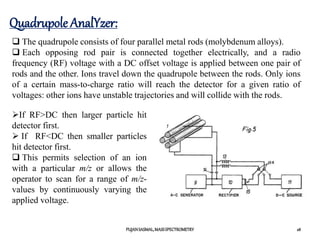
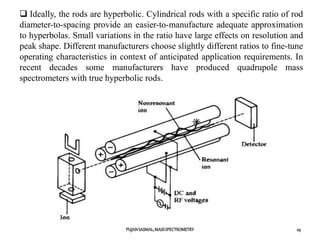
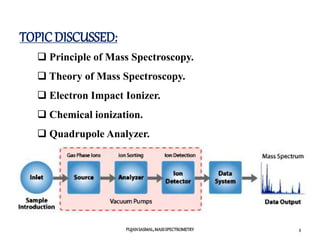
The document discusses mass spectrometry, including:
1) The principles of mass spectrometry which involve ionizing samples and separating ions based on their mass-to-charge ratio.
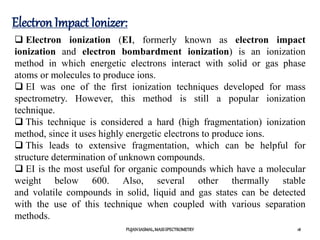
2) Different ionization techniques like electron impact ionization which uses energetic electrons to produce ions.
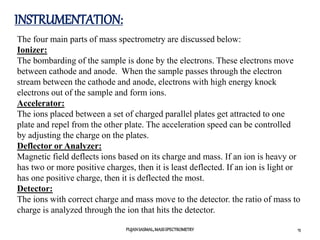
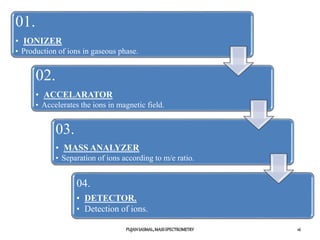
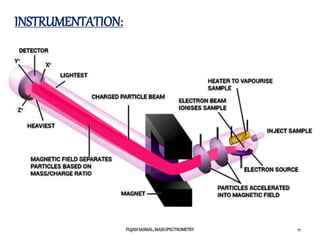
3) The typical instrumentation of a mass spectrometer including an ionizer, accelerator, mass analyzer, and detector.
4) A brief history of developments in mass spectrometry from the late 19th century onward.





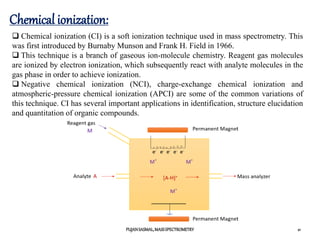
![Chemical ionization:
PUJANSASMAL,MASSSPECTROMETRY 22
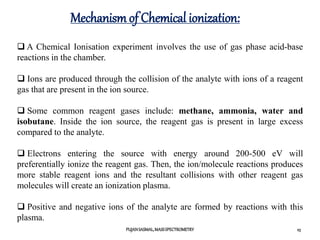
Chemical ionization requires a lower amount of energy compared to electron
ionization (EI), but this depends on the reactant material used.
This low-energy ionization mechanism yields less or sometimes no
fragmentation, and usually a simpler spectrum.
The lack of fragmentation limits the amount of structural information that can
be determined about the ionized species.
However, a typical CI spectrum has an easily identifiable protonated molecular
ion peak [M+1]+, which allows easy determination of molecular mass.
This technique requires the transfer of high-mass entities from the reagent gas
to the analyte, and therefore, the Franck-Condon principle does not govern the
process of ionization. CI is thus quite useful in cases where the energy of the
bombarding electrons in EI is high, resulting exclusively in fragmentation of the
analyte, causing the molecular-ion peak to be less detectable or completely
absent.](https://image.slidesharecdn.com/finalmassspectrometry-210301101952/85/Mass-Spectrometry-22-320.jpg)