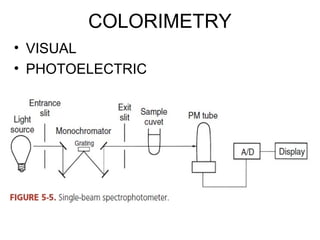
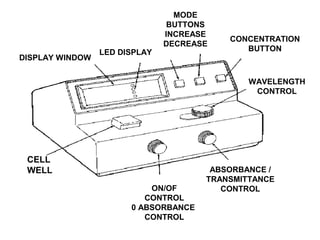
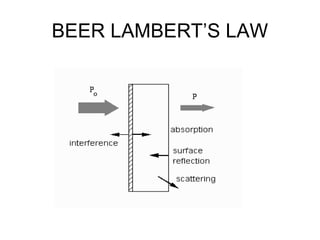
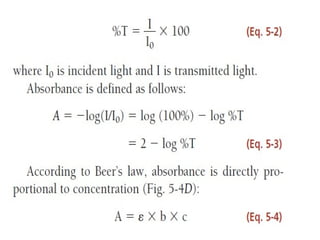
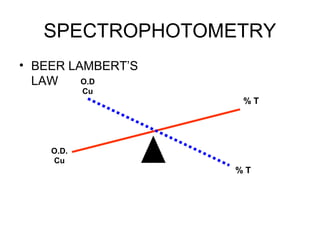
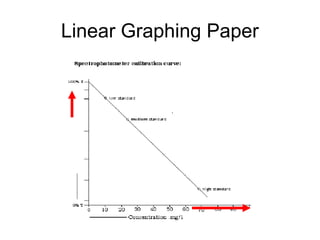
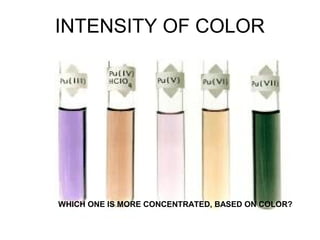
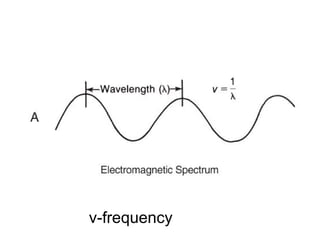
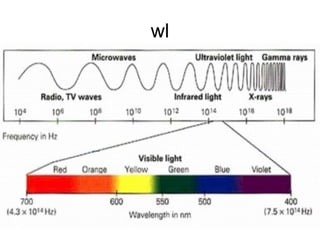
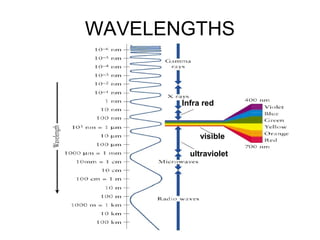
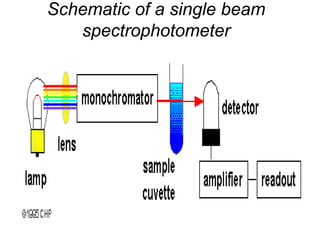
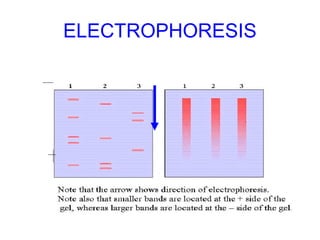
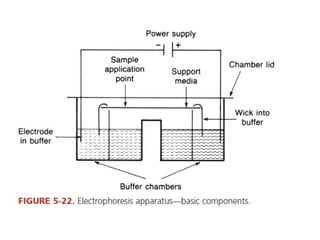
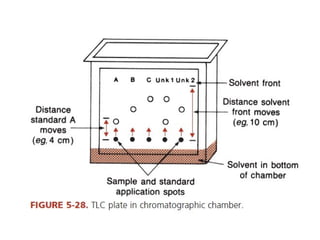
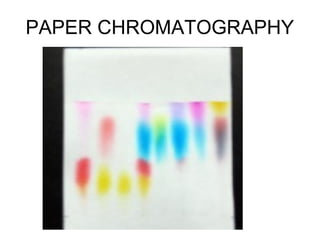
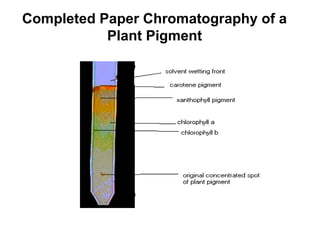
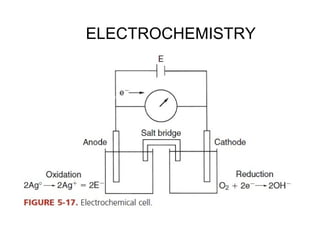
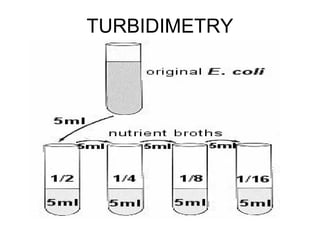
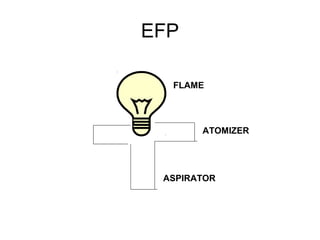
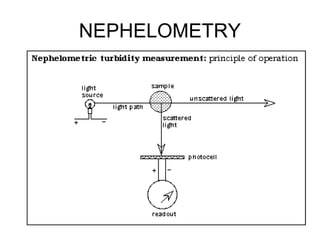
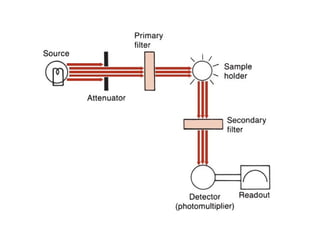
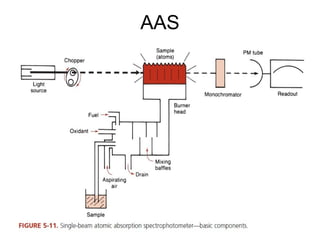
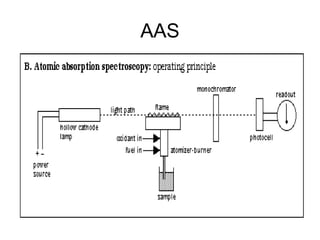
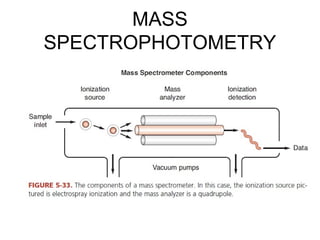
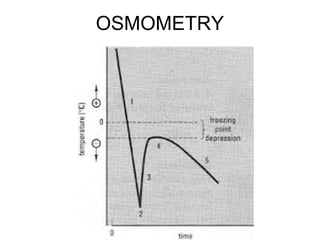
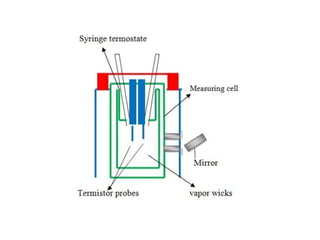
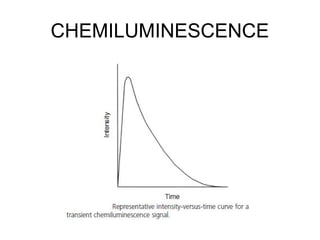
This document discusses various analytical instrumentation and methods. It begins by explaining the objectives of the lecture, which are to explain the principles of methods, discuss factors that may affect the methods, and know the importance of the methods. It then discusses several analytical techniques including colorimetry, spectrophotometry, electrophoresis, chromatography, electrochemistry, and atomic absorption spectroscopy. It provides examples and diagrams to illustrate the principles and components involved in these important analytical methods.