This document discusses dry chemistry techniques. It begins with a brief history, noting the first dry chemistry system for testing urine sugar in 1941. The key was using dried ingredients and controlling humidity.
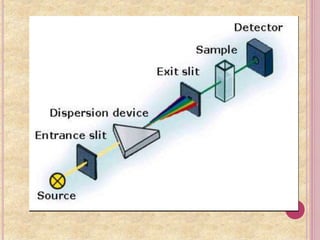
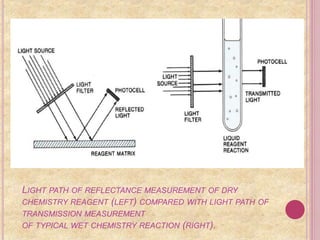
It then explains the principle of dry chemistry is based on reflectance spectrophotometry. Dry chemistry components use reflectance to measure color changes rather than transmission used in wet chemistry.
Examples of dry chemistry tests for urine analysis using reagent strips are provided, detecting substances like glucose, protein, blood, and pH. Dry chemistry is also used in blood tests measuring analytes like creatinine and uric acid.





































![MAINTENANCE
Dust may change the whiteness of sphere so keep the
room dust free and clean the sphere from time to time.
Proper electric stabilization is required.
The ambient temperature and humidity should be
properly regulated.
Do not touch used slides with bare hands as this may
cause contamination.
The slides should be stored in a refrigerator [2-8 ºC
(35.6-46.4 ºF)] without unwrapping to avoid humidity,
light, and heat.
Measurement should be completed within 30 minutes
after unwrapping the individual package.
A new slide must be used for each measurement.](https://image.slidesharecdn.com/drychemistry-200903204651/85/Dry-chemistry-41-320.jpg)
