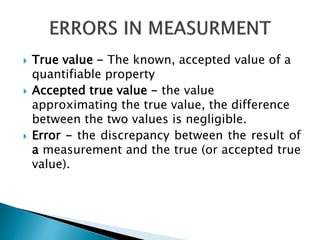
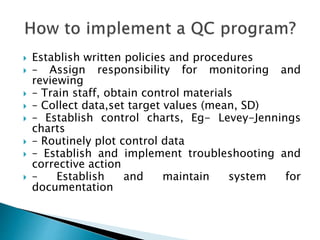
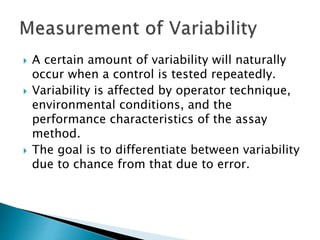
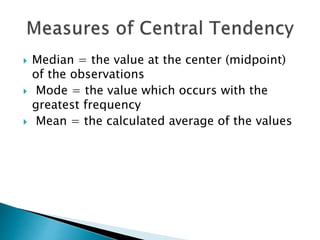
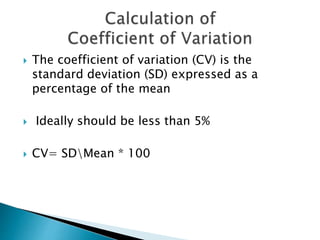
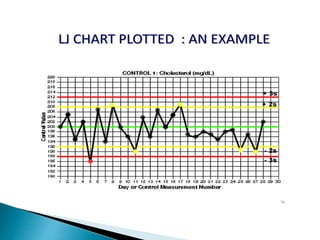
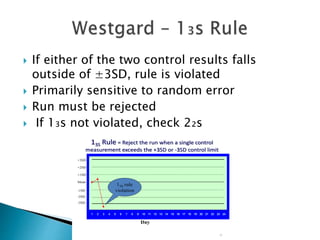
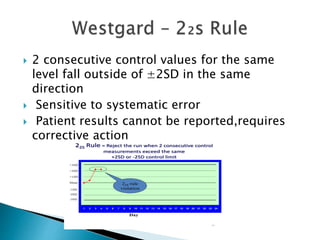
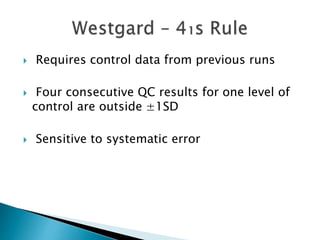
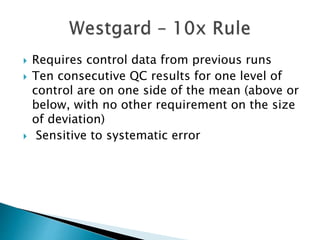
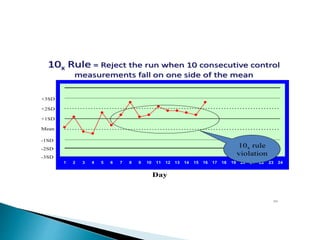
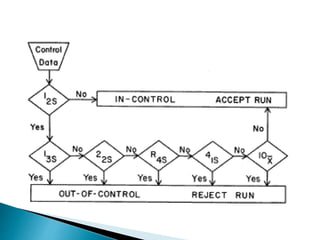
The document discusses various quality control procedures that are important for ensuring accurate and reliable laboratory test results. It covers topics like calibration checks, control charts, quality control rules, corrective actions, and external quality assessment. The key aspects emphasized are the need for documented quality control protocols, monitoring control results for errors, and taking actions to correct any issues identified.