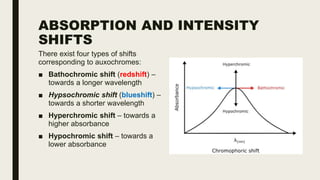
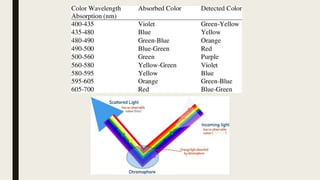
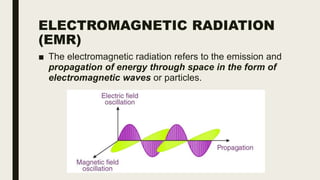
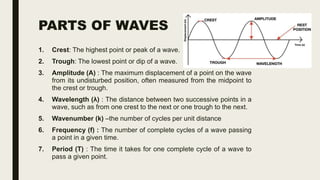
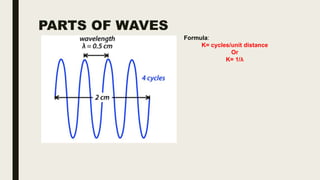
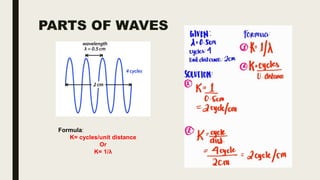
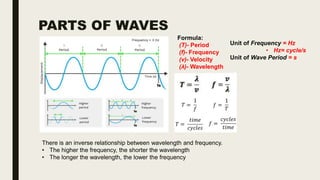
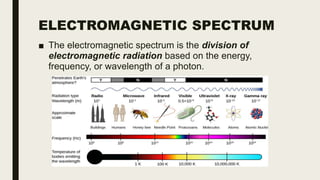
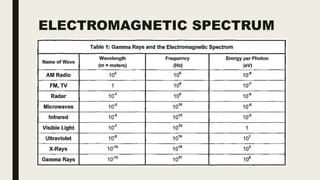
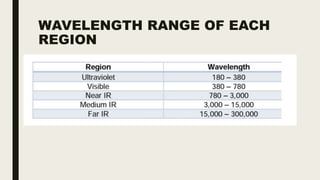
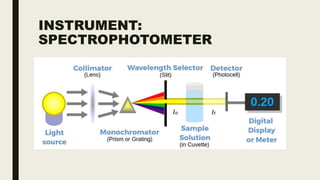
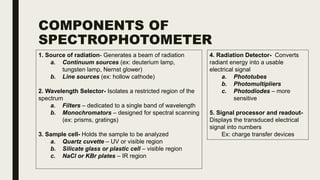
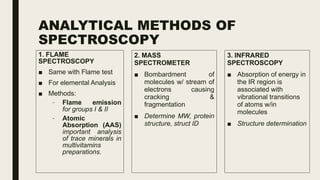
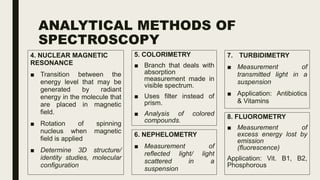
This document discusses various spectroscopic methods used for official assay. It describes instrumental methods like spectrometric and chromatographic techniques that use instruments to measure physicochemical properties. Spectrometric methods are discussed in detail, including how spectroscopy works via absorption or emission of electromagnetic radiation when interacting with matter. Key components of a spectrophotometer and analytical spectroscopic techniques are also outlined, such as atomic absorption spectroscopy, infrared spectroscopy, and nuclear magnetic resonance spectroscopy.