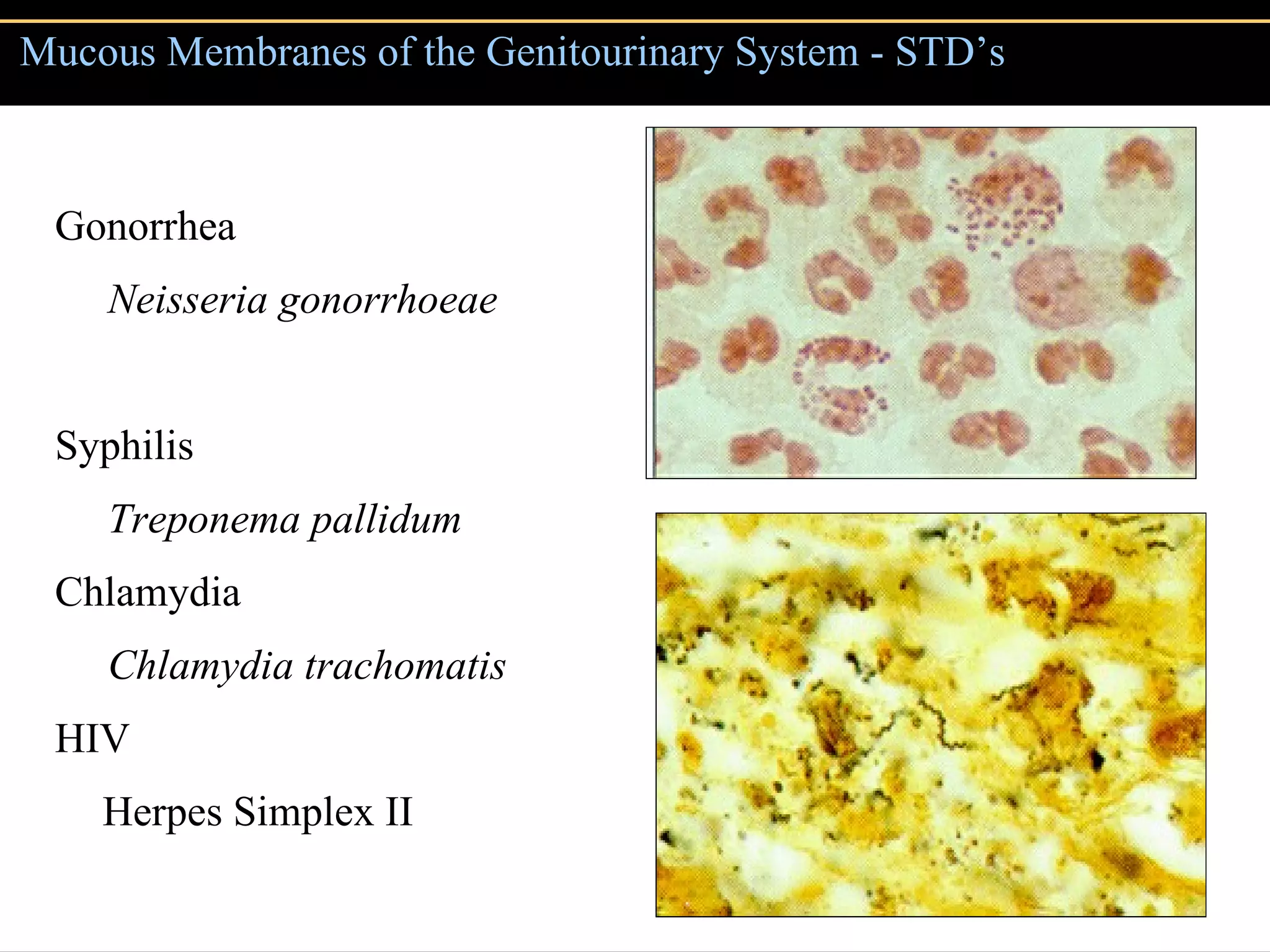
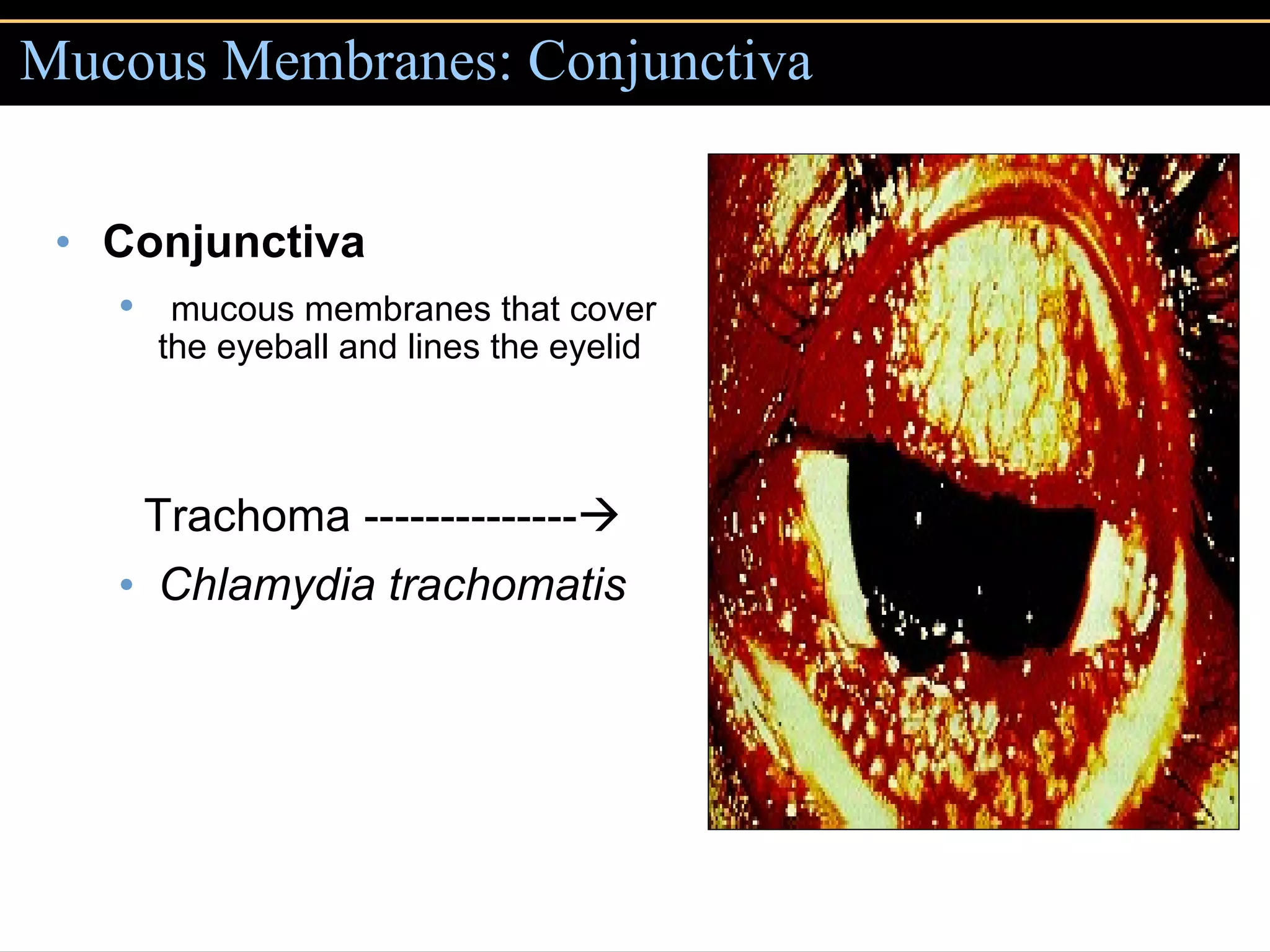
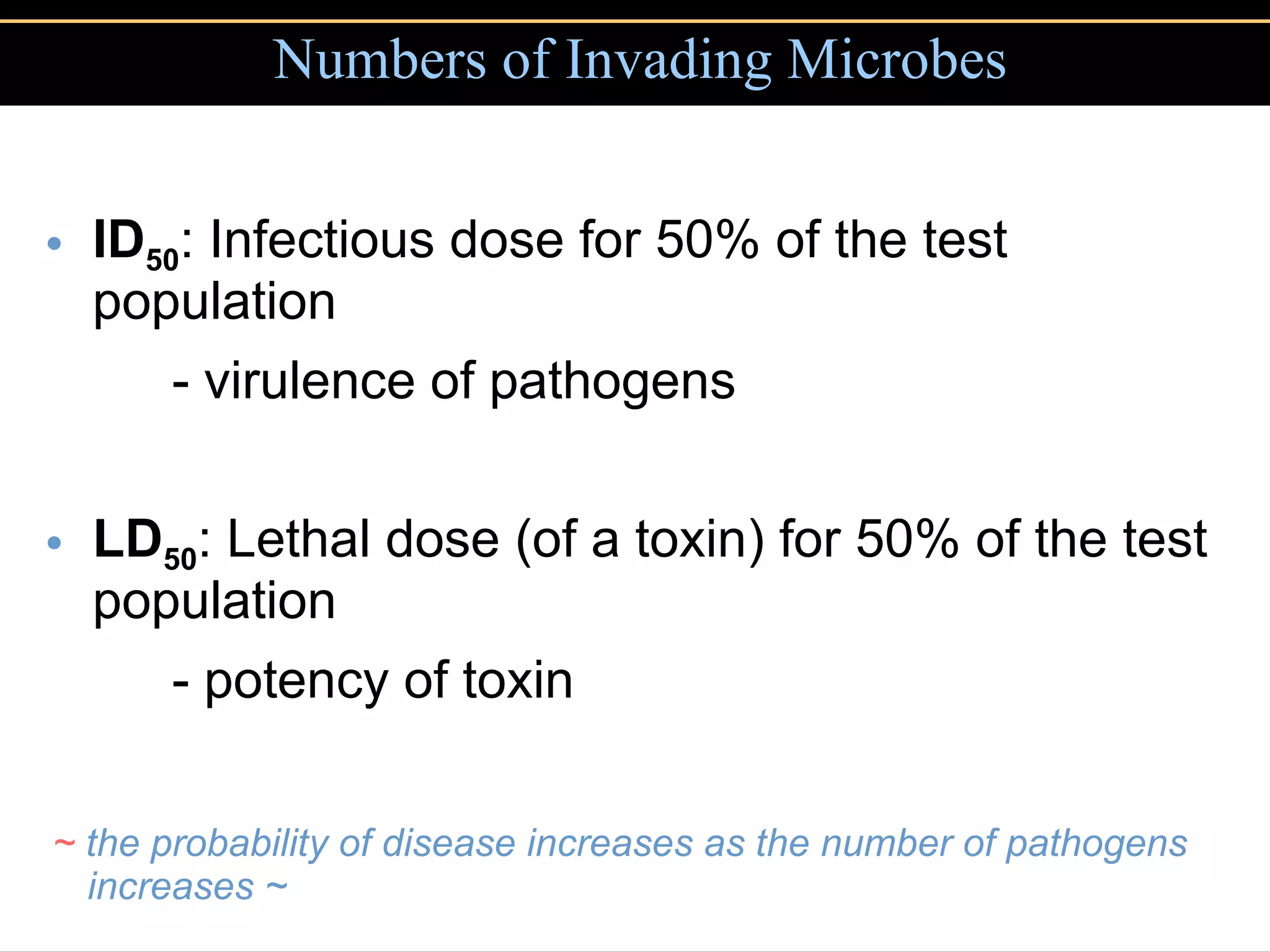
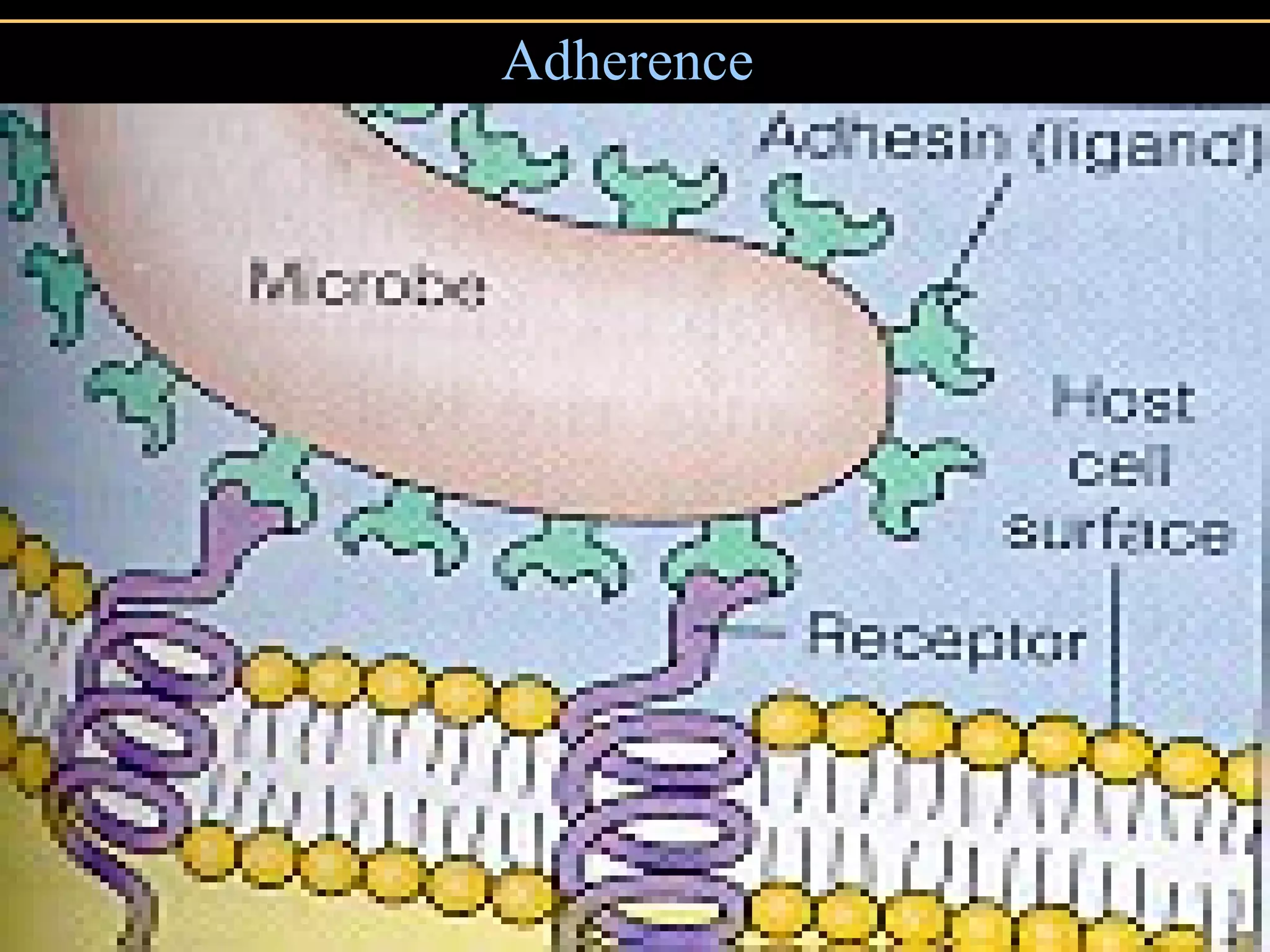
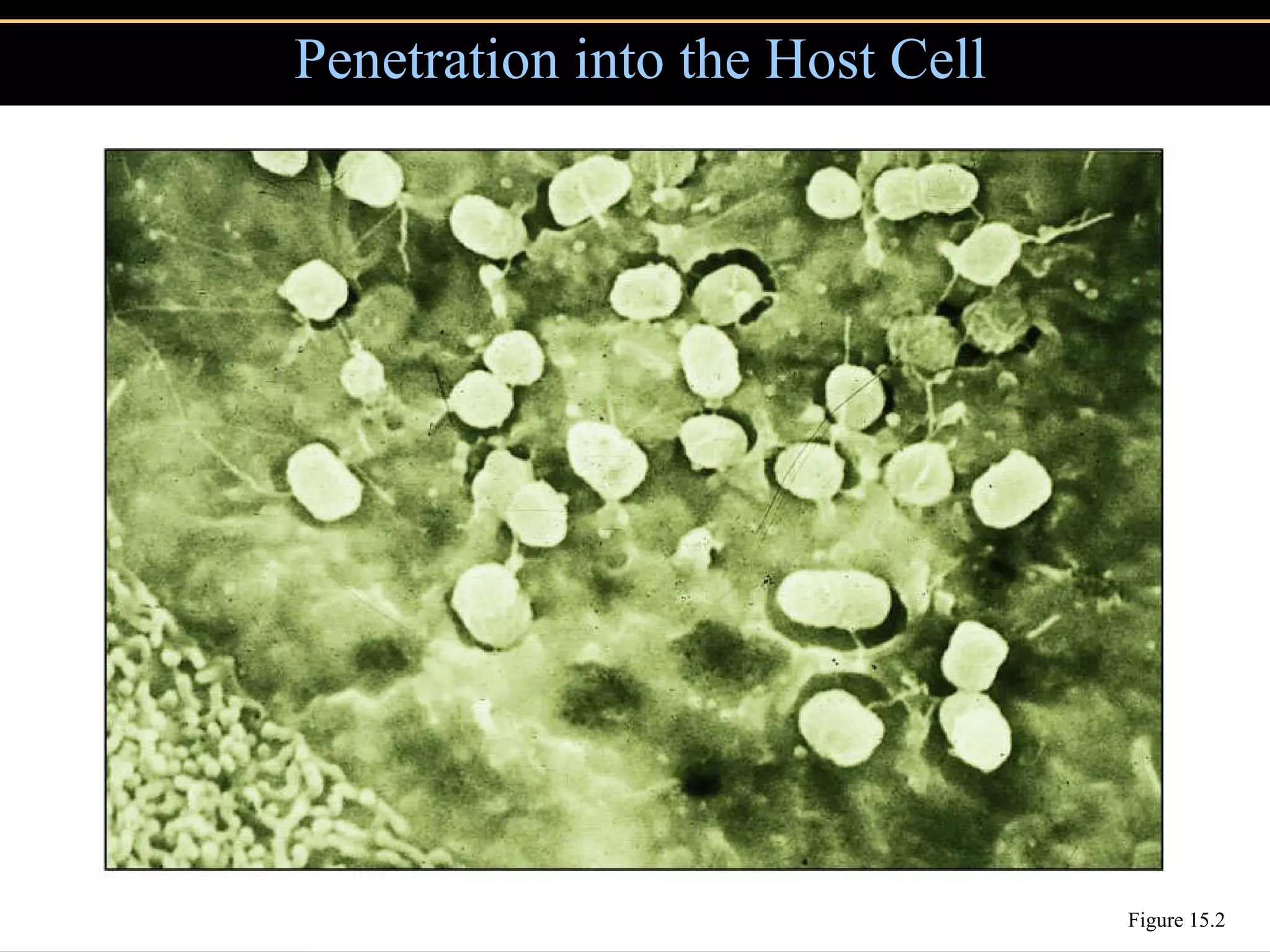
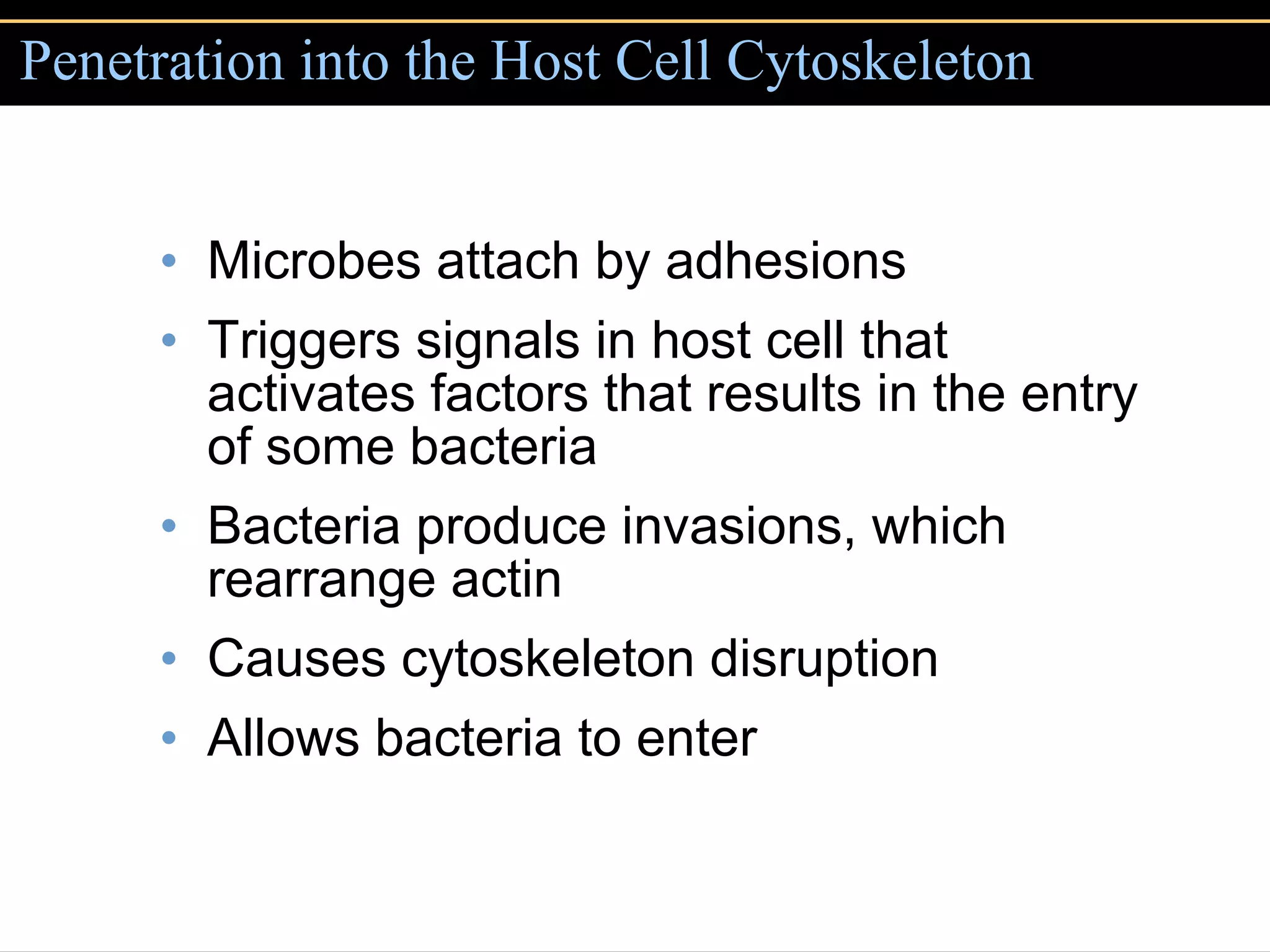
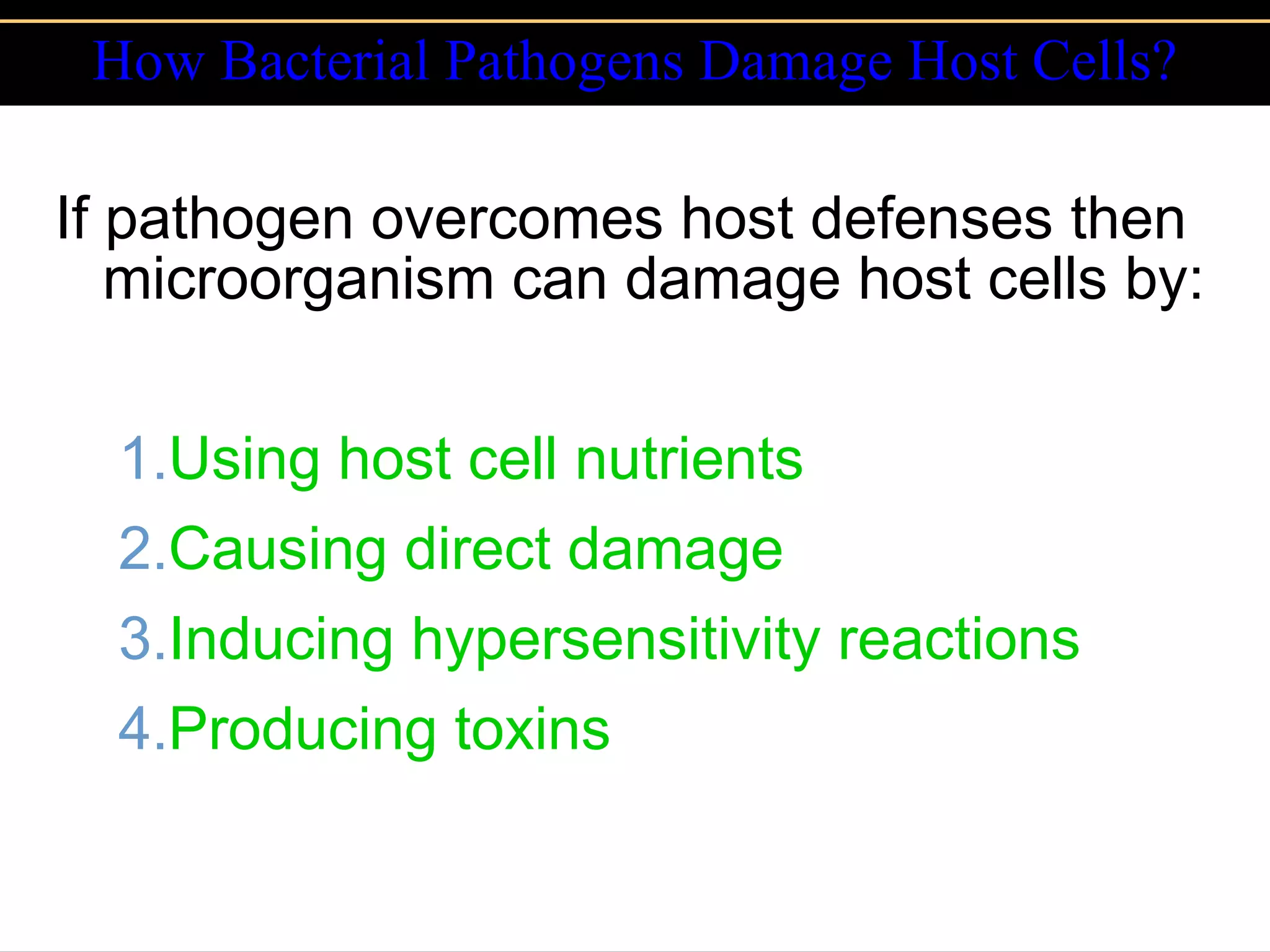
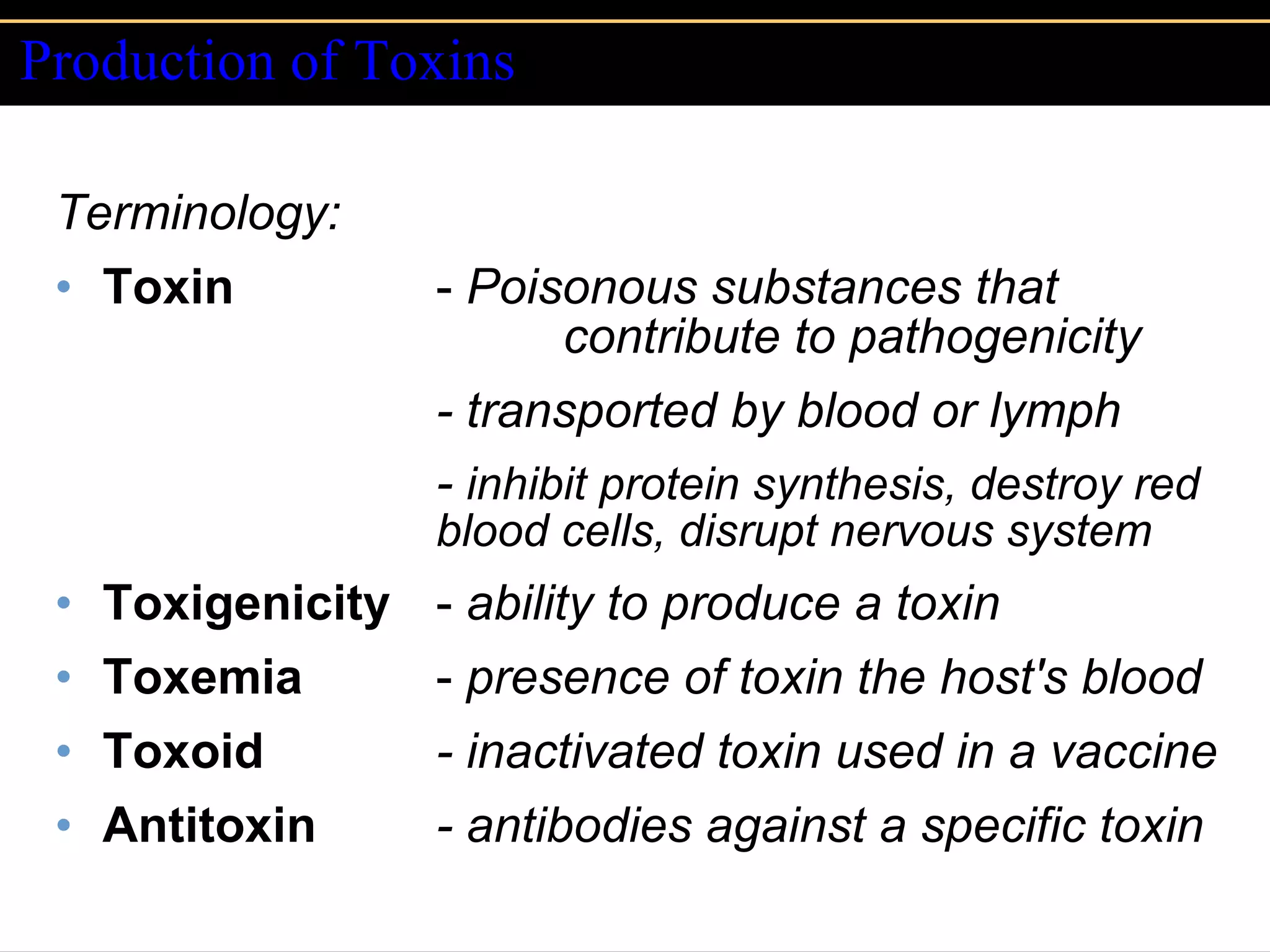
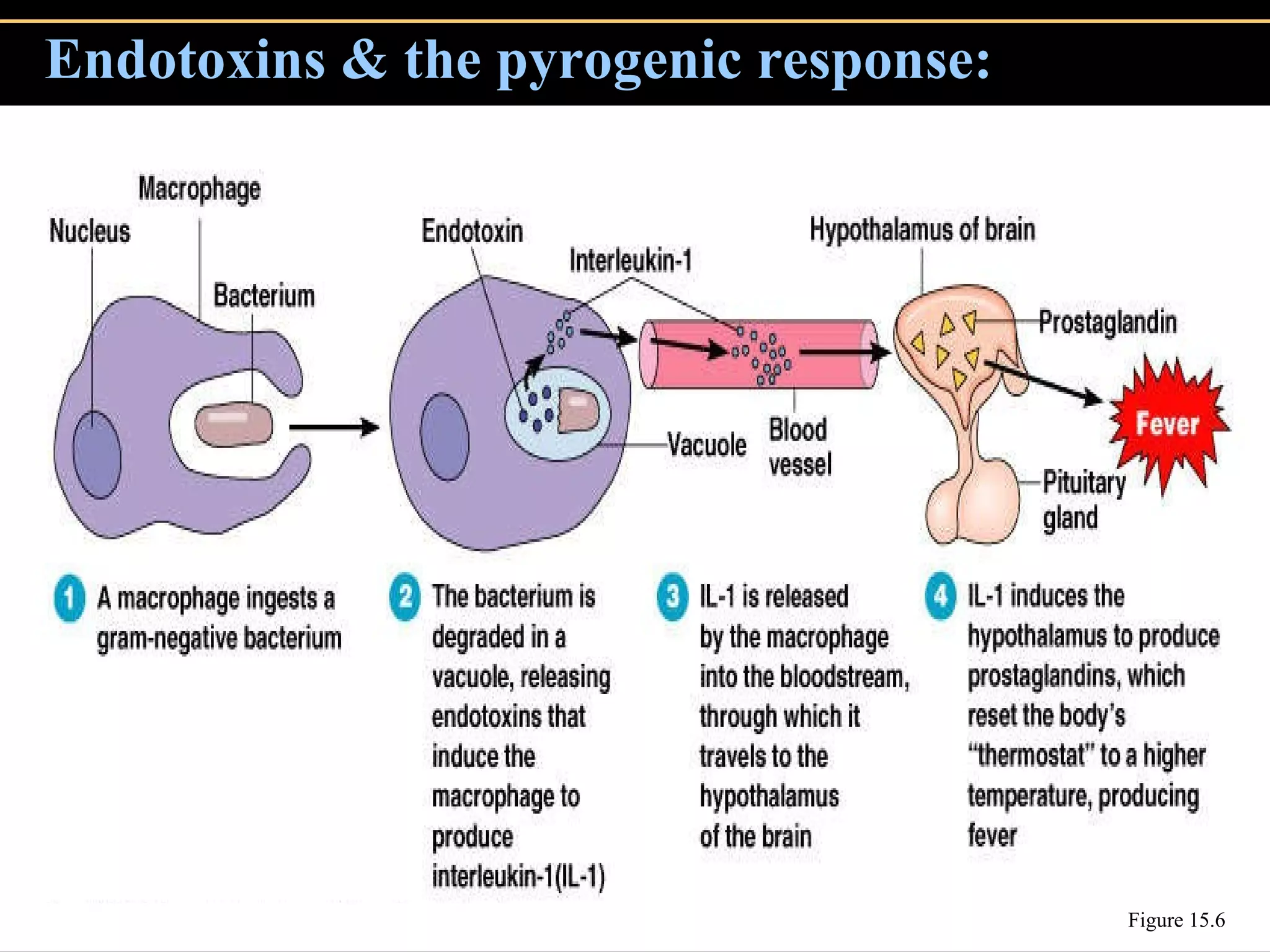
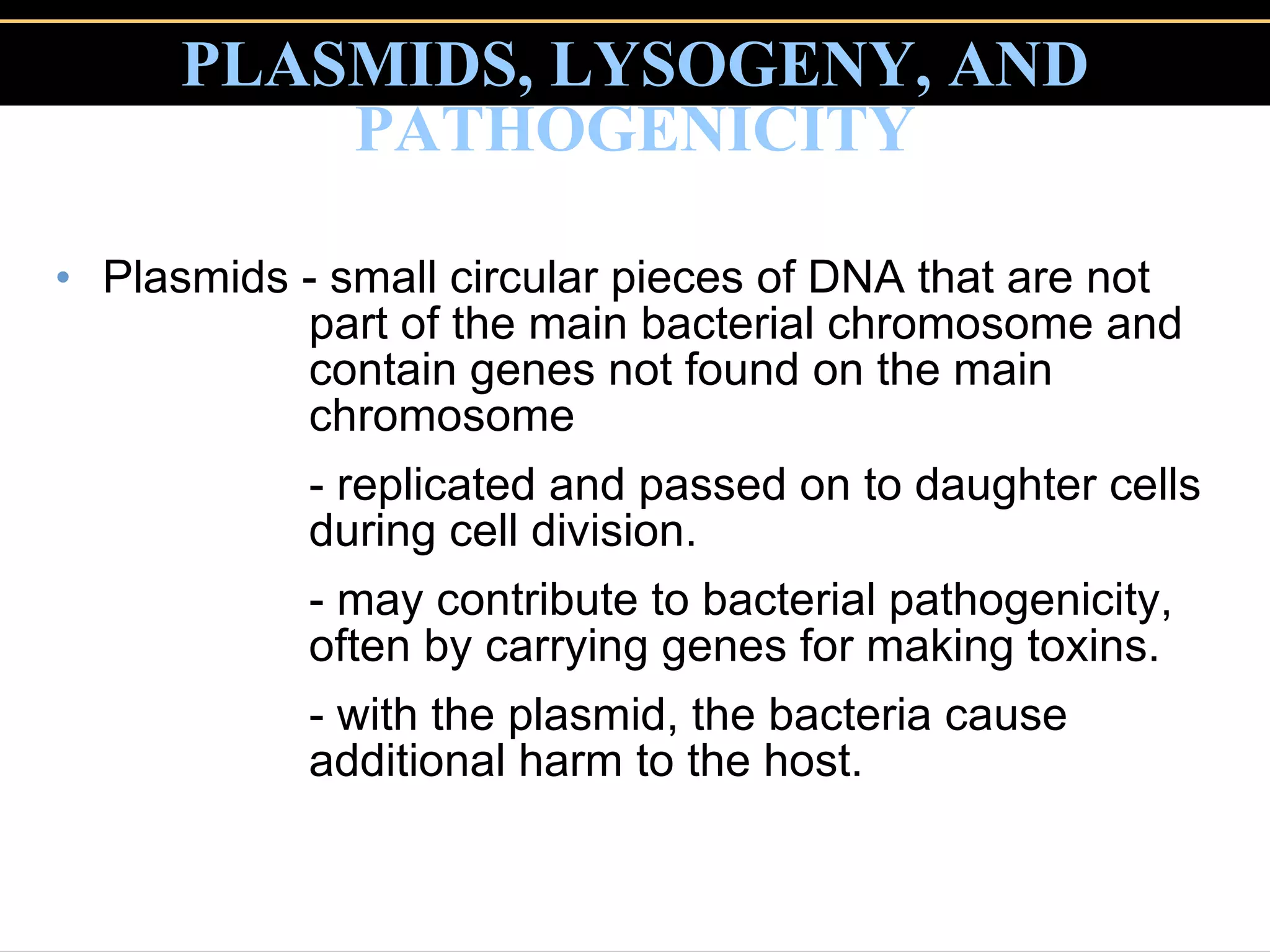
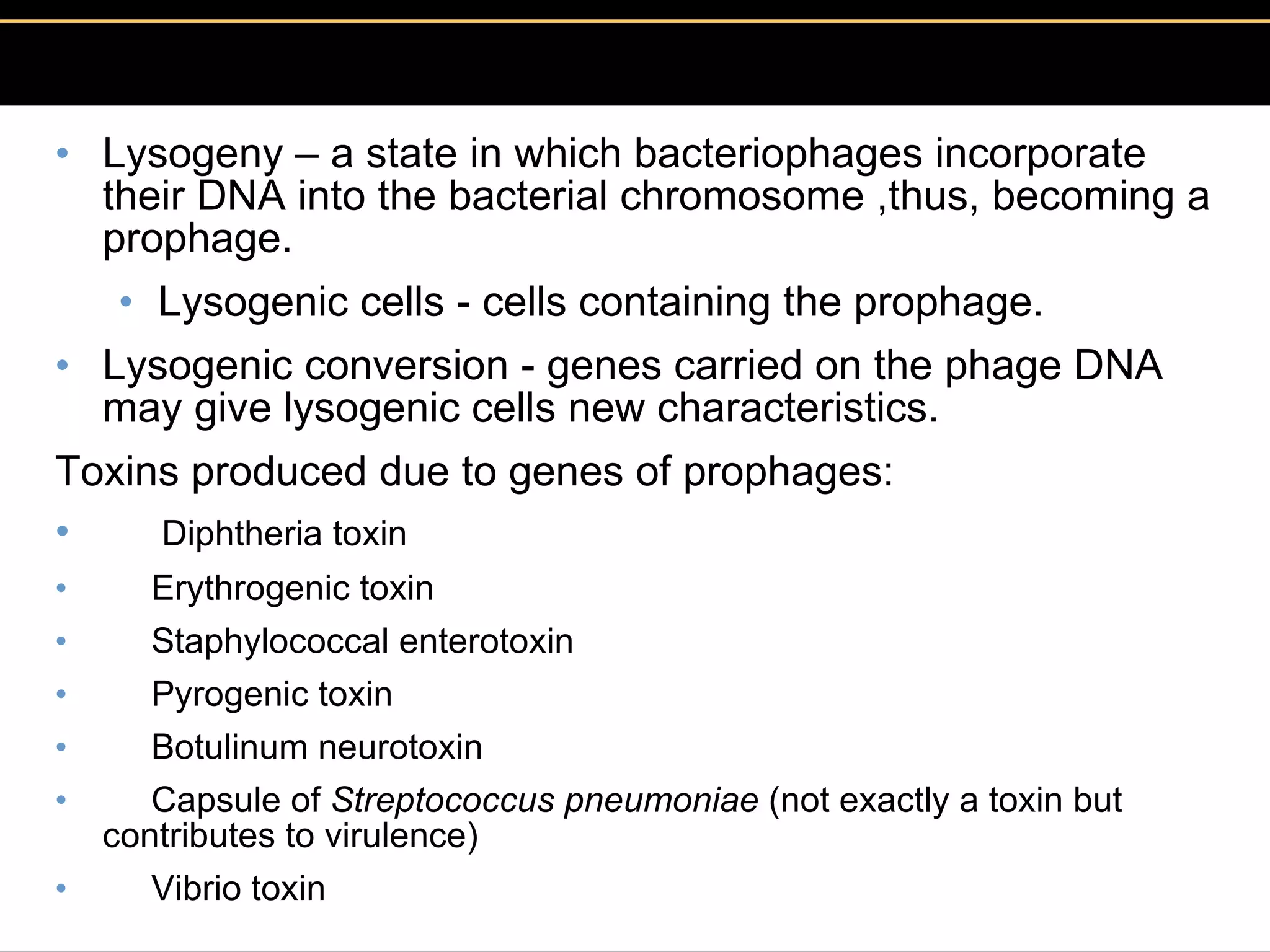
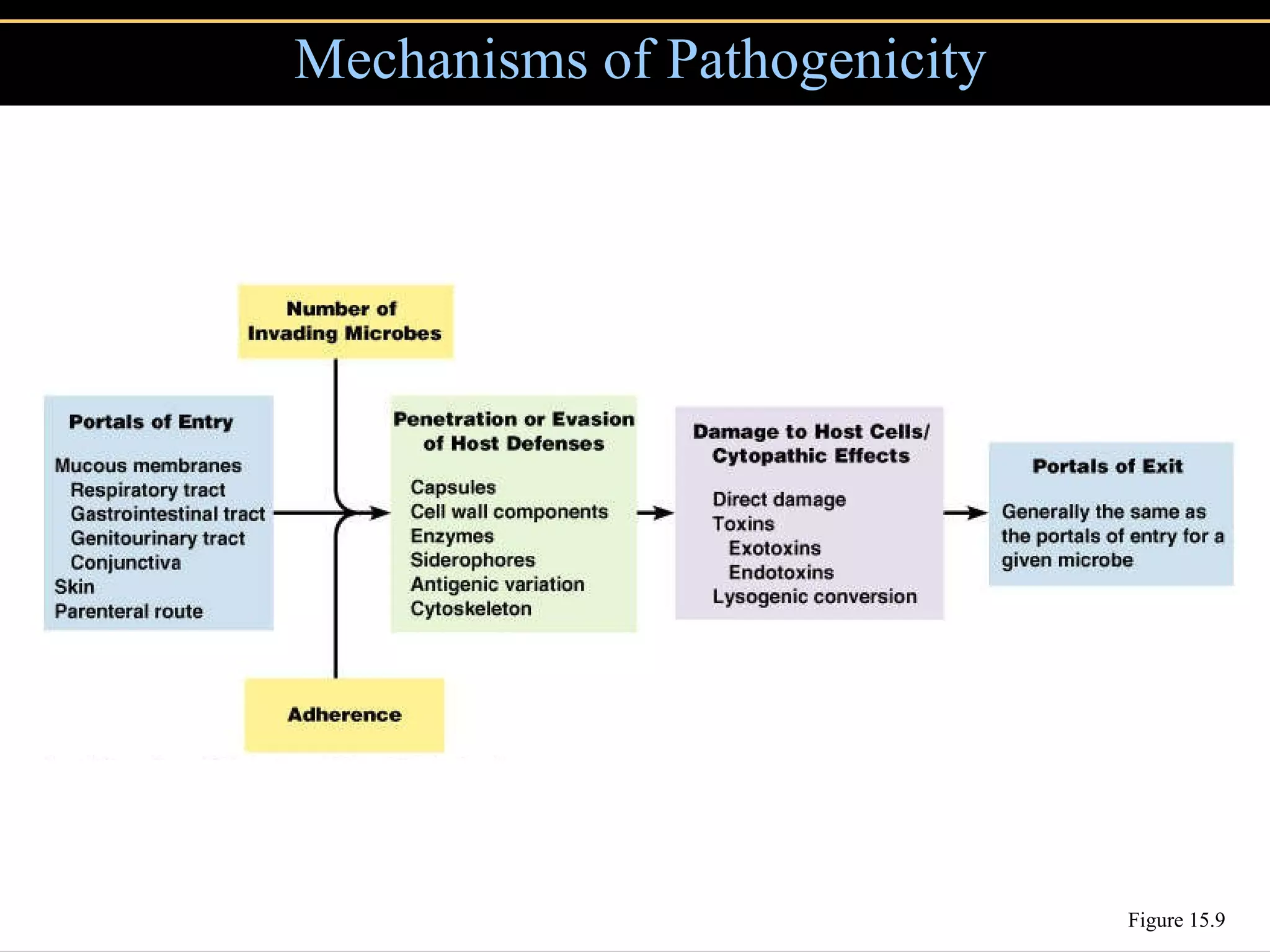
Microbial pathogens can cause disease through several mechanisms:
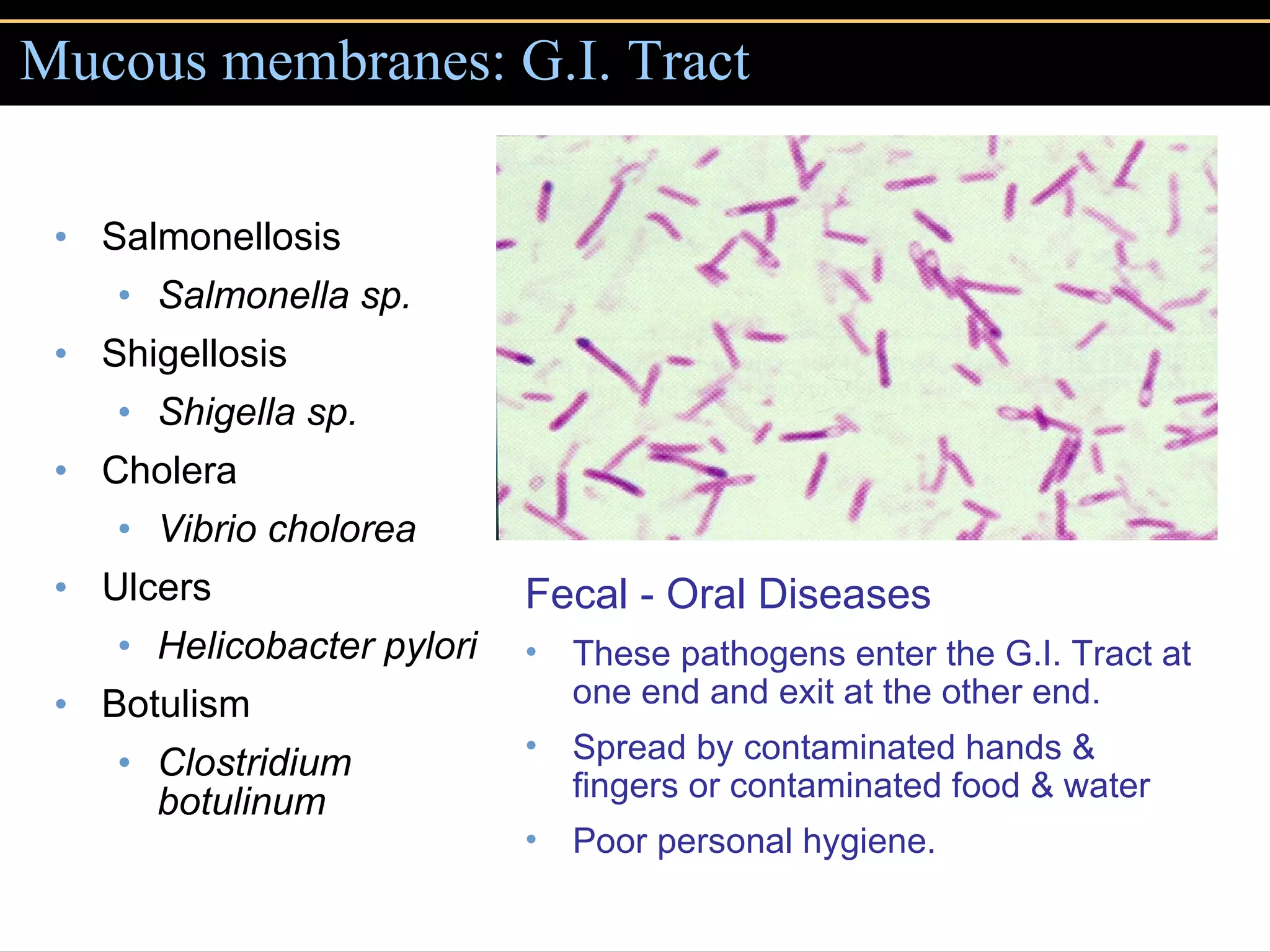
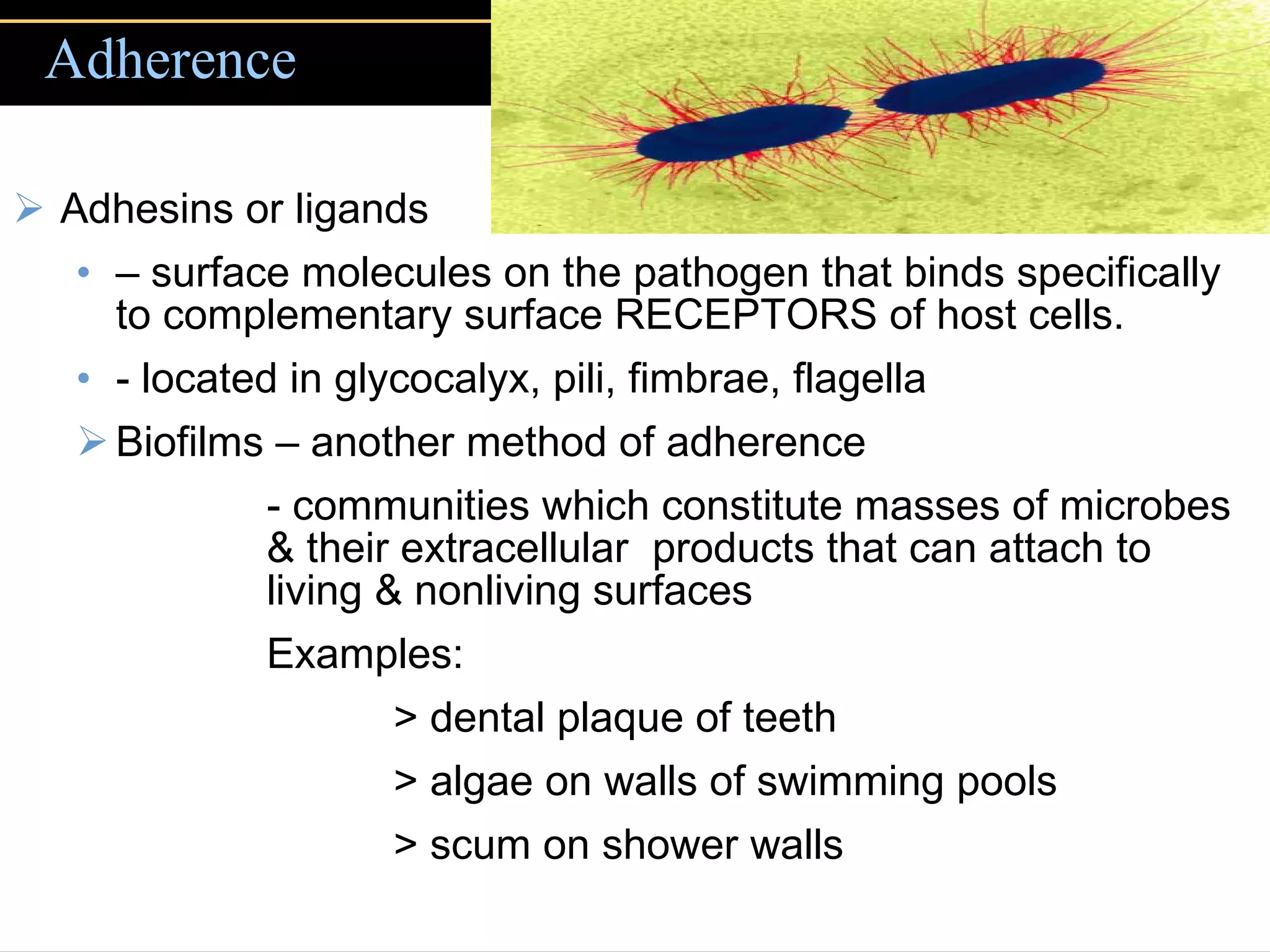
1. They gain entry into the host through portals of entry like mucous membranes or skin and adhere using adhesins.
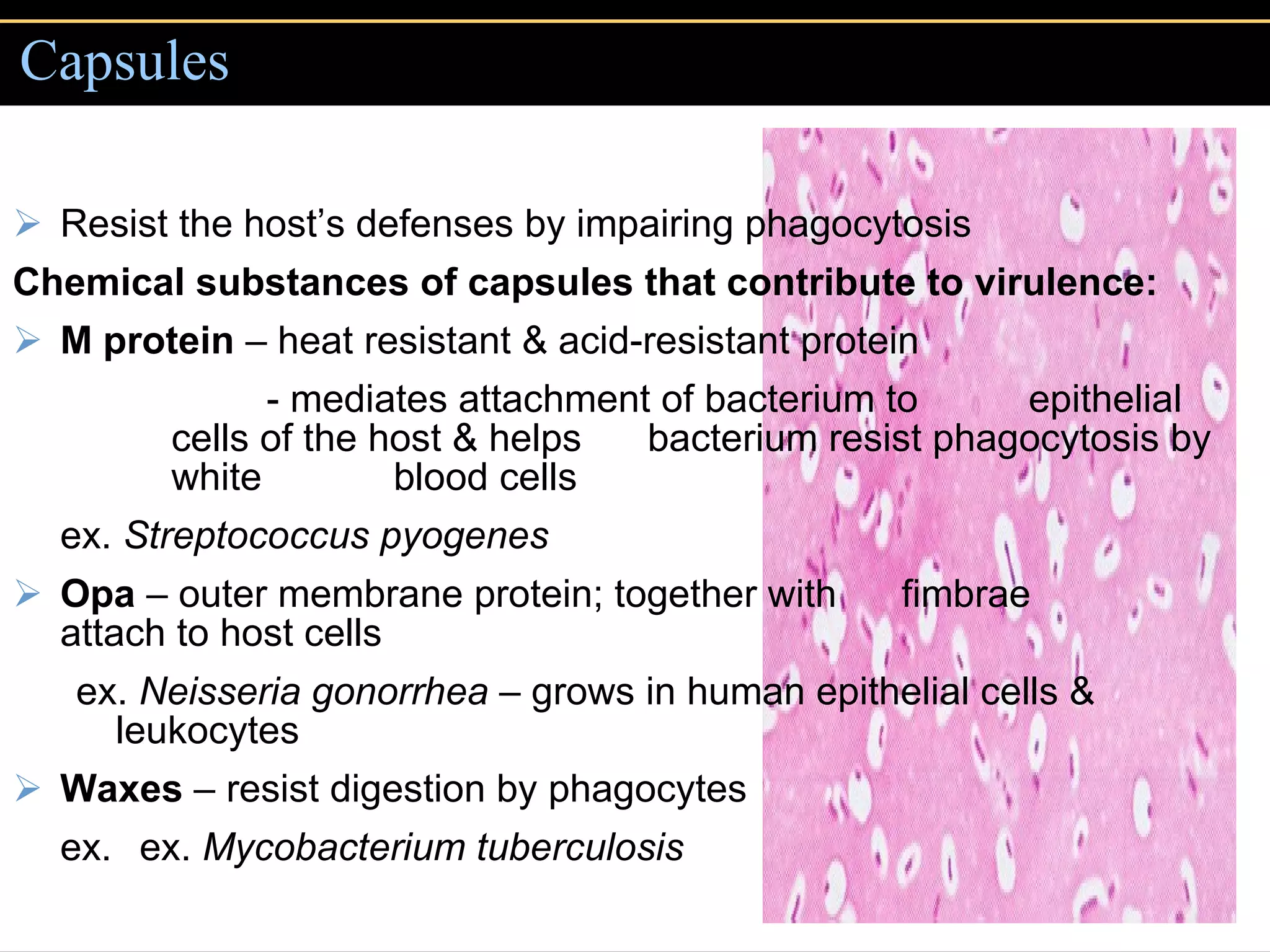
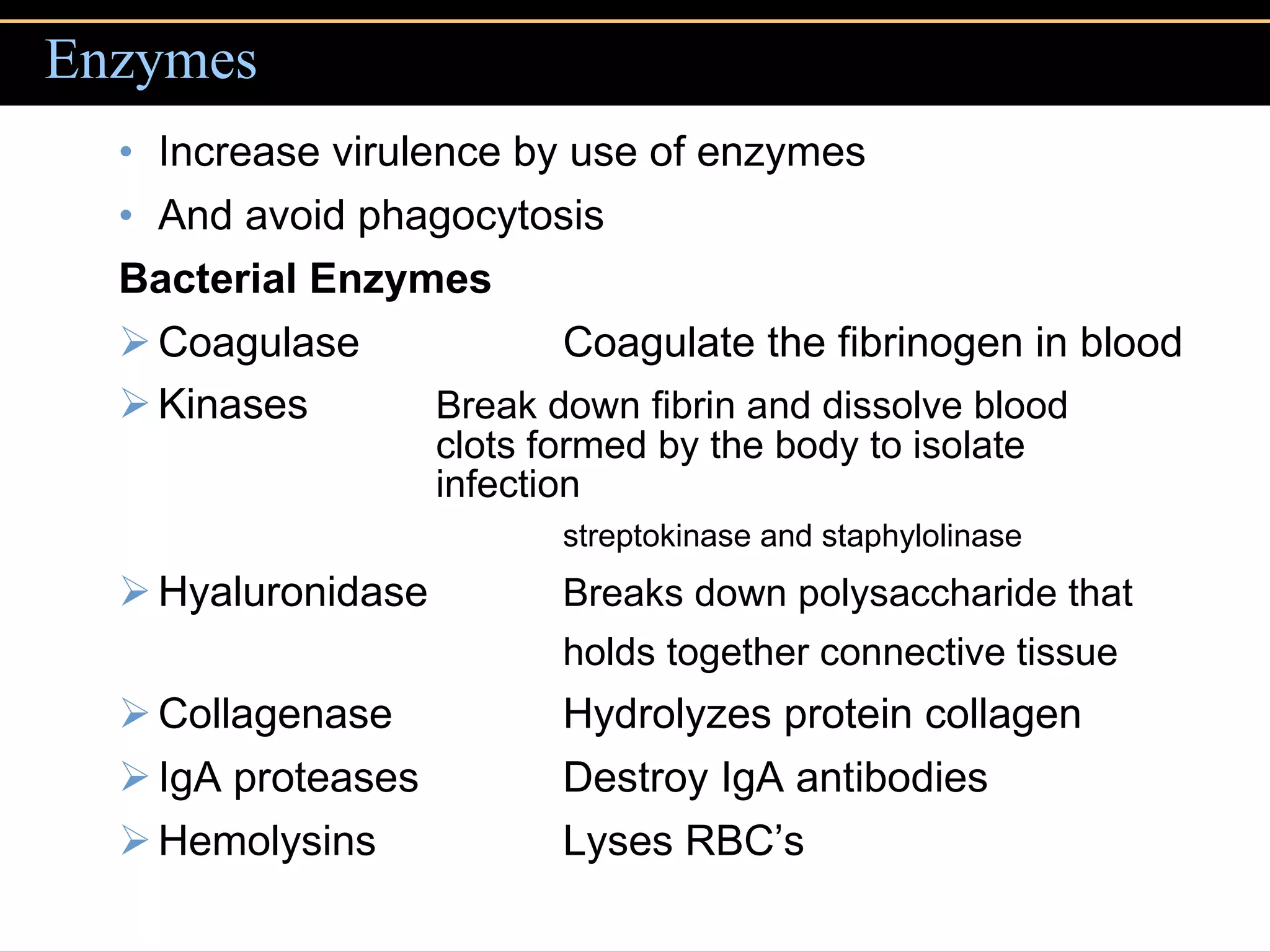
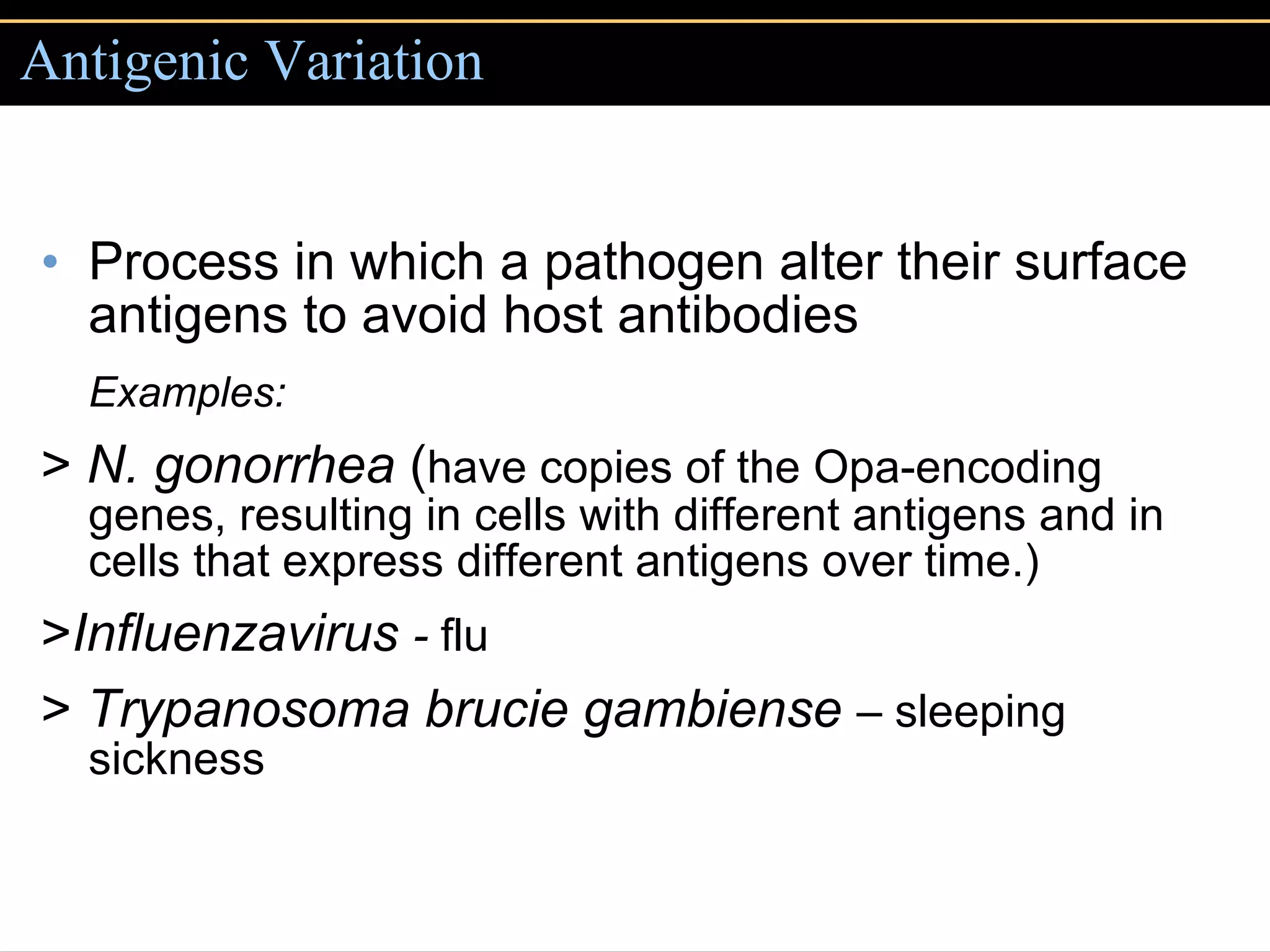
2. To overcome host defenses, they avoid phagocytosis using capsules or enzymes and penetrate host cells.
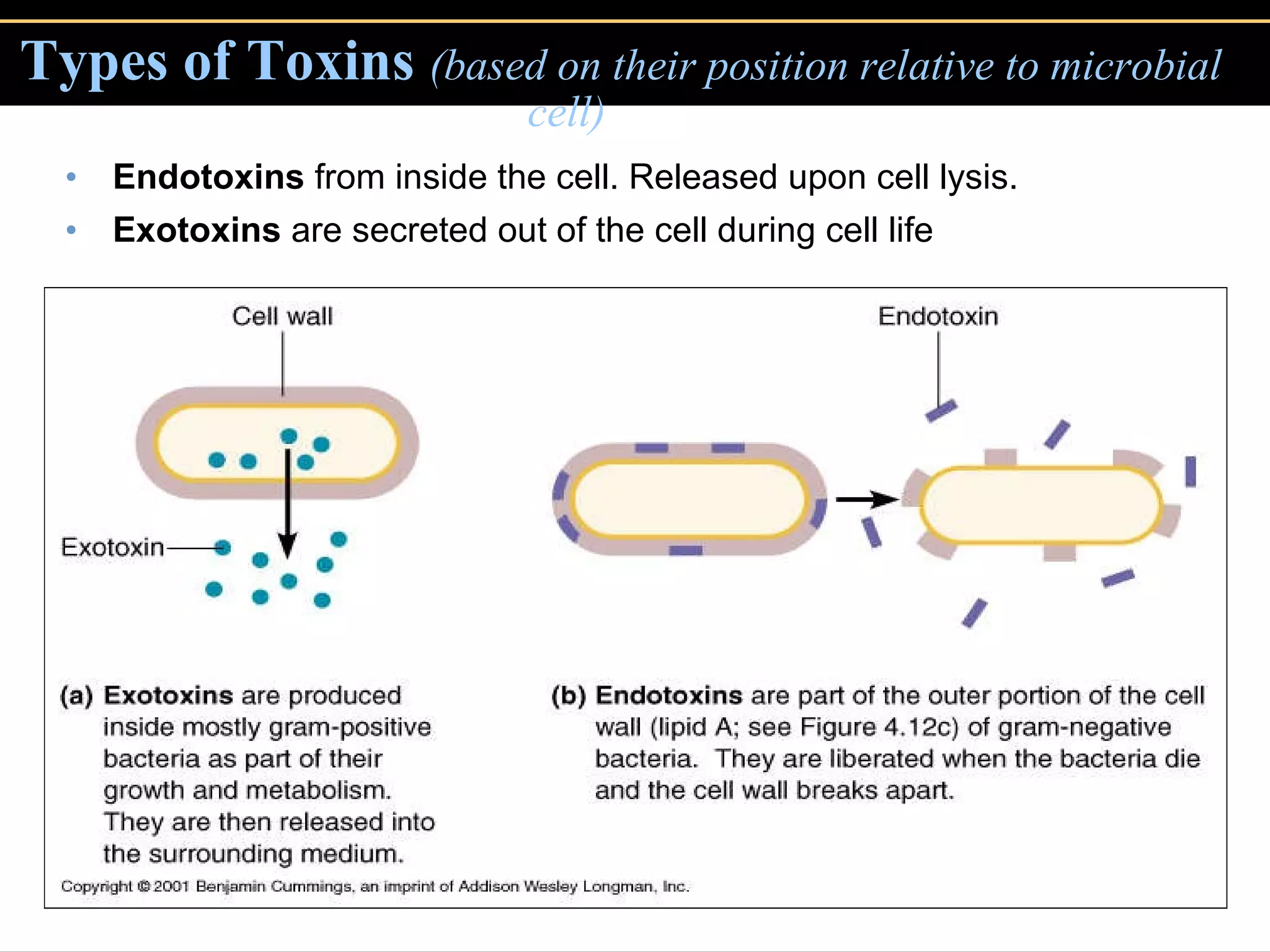
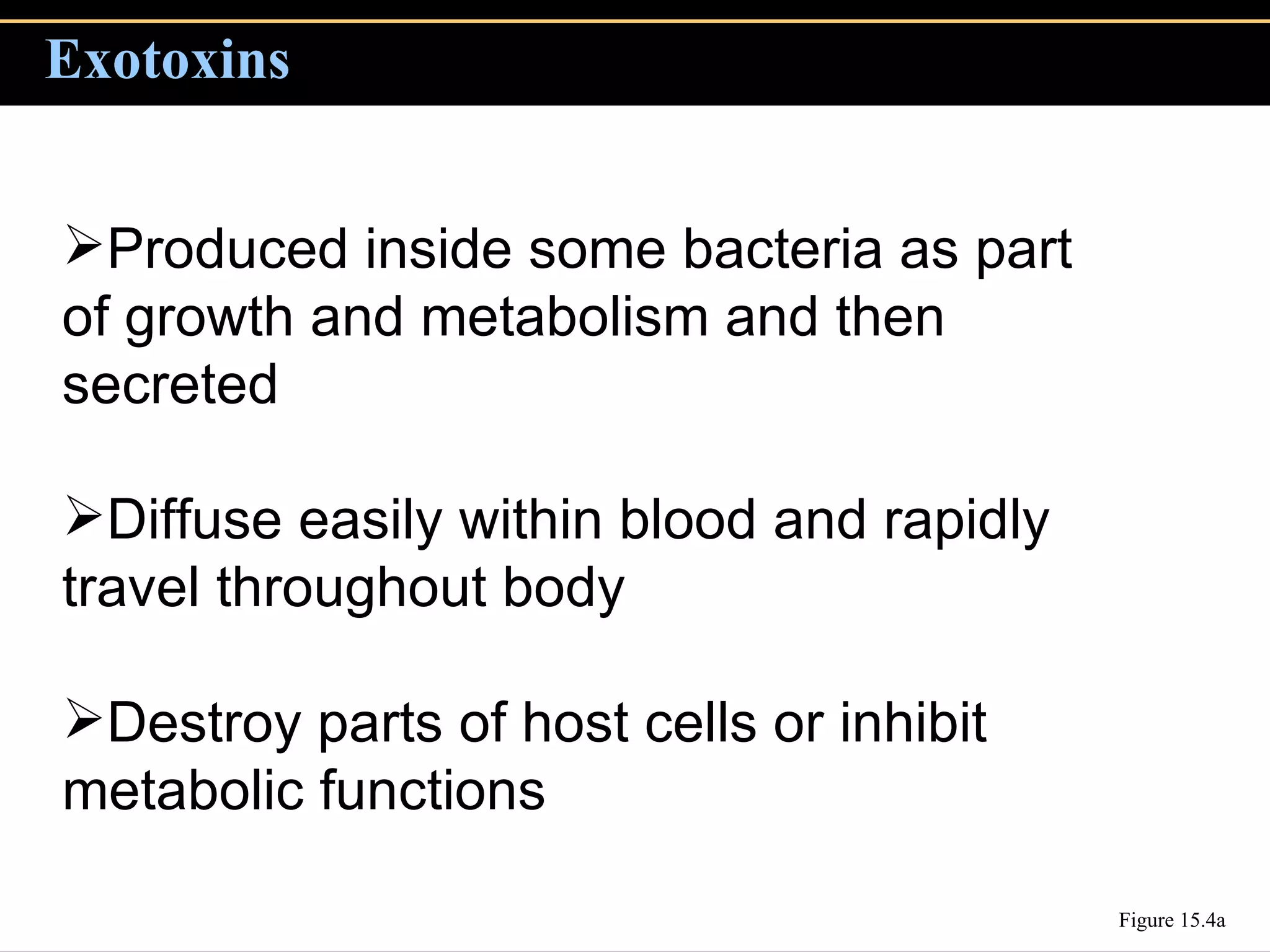
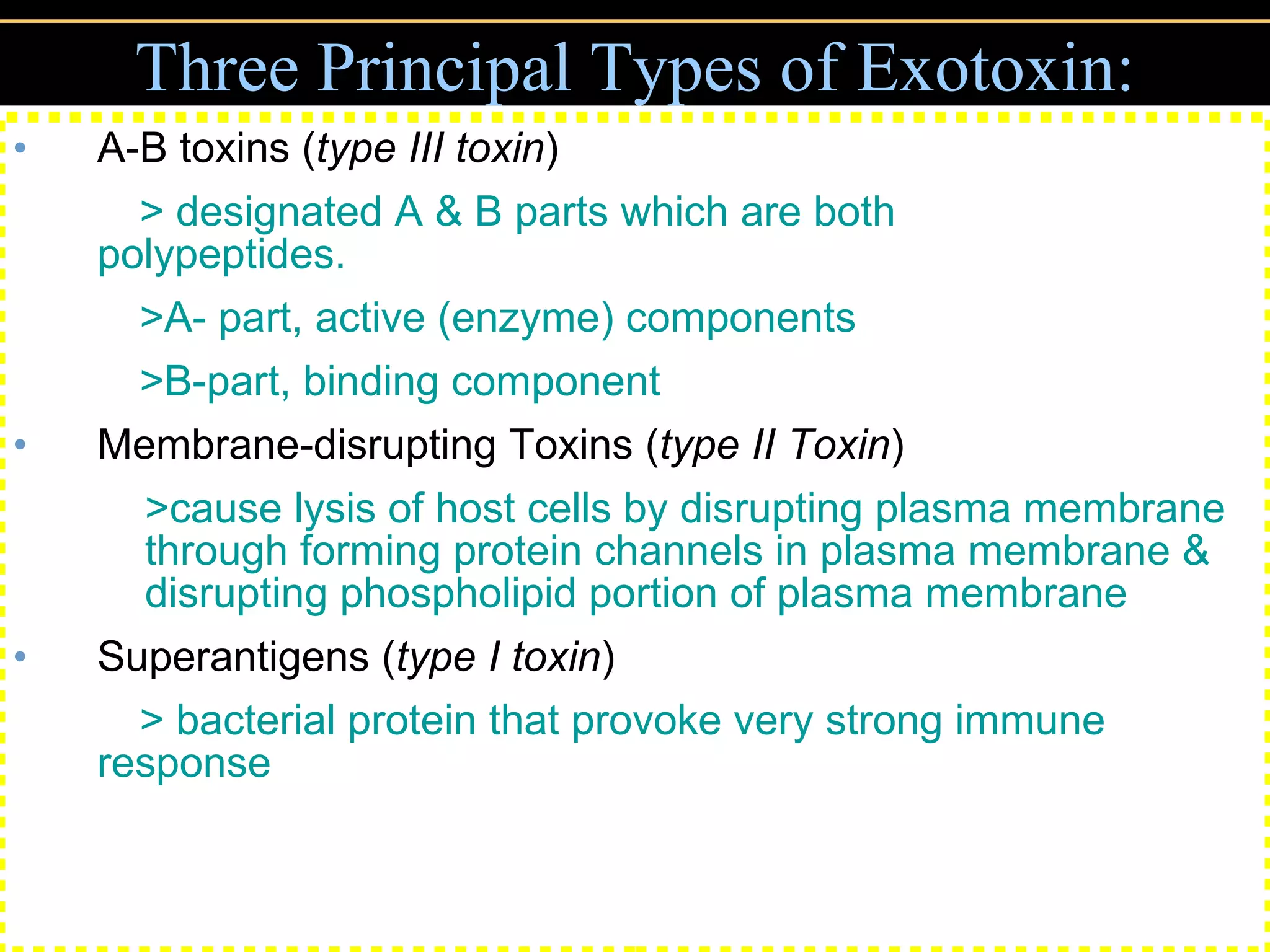
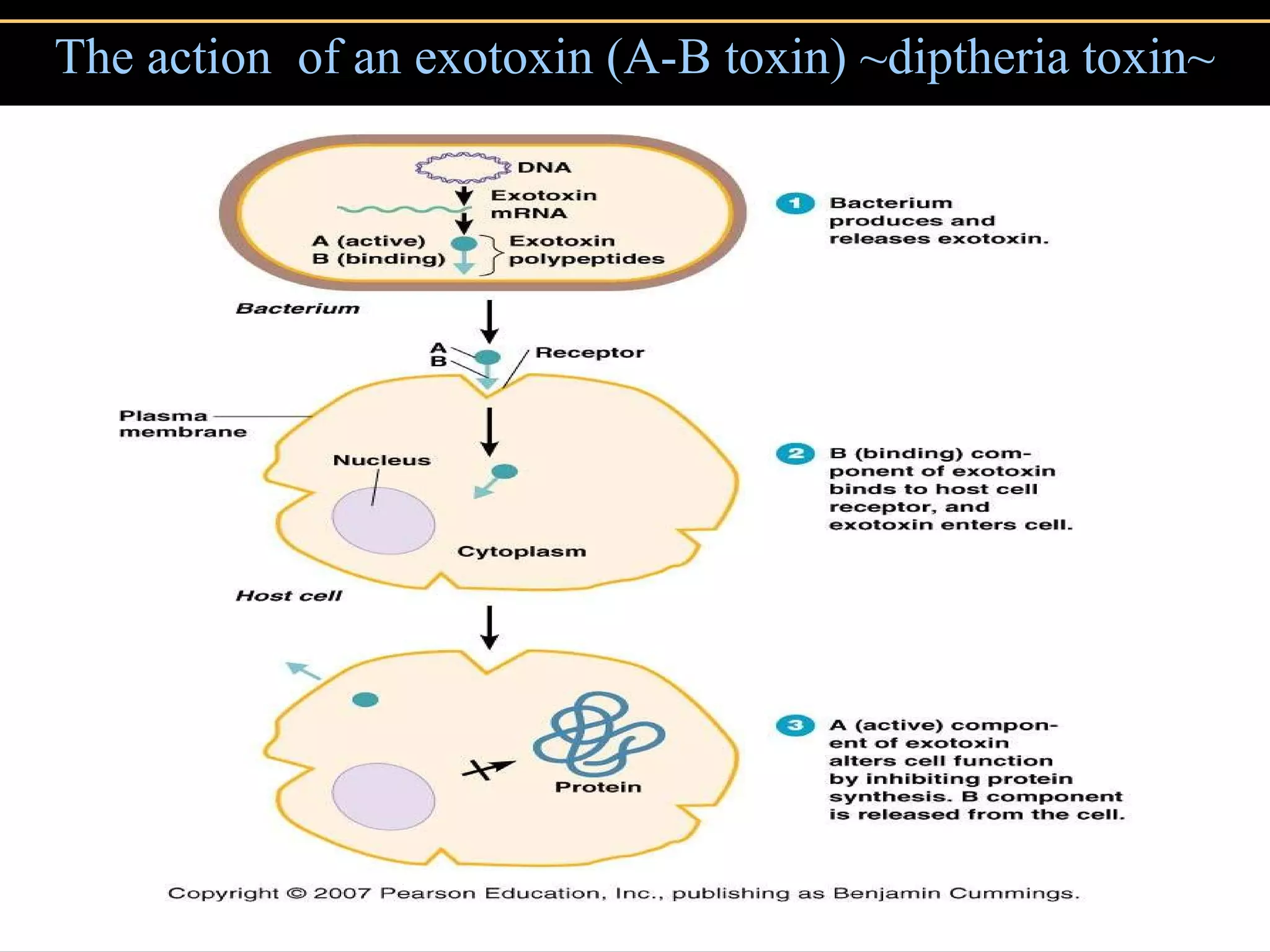
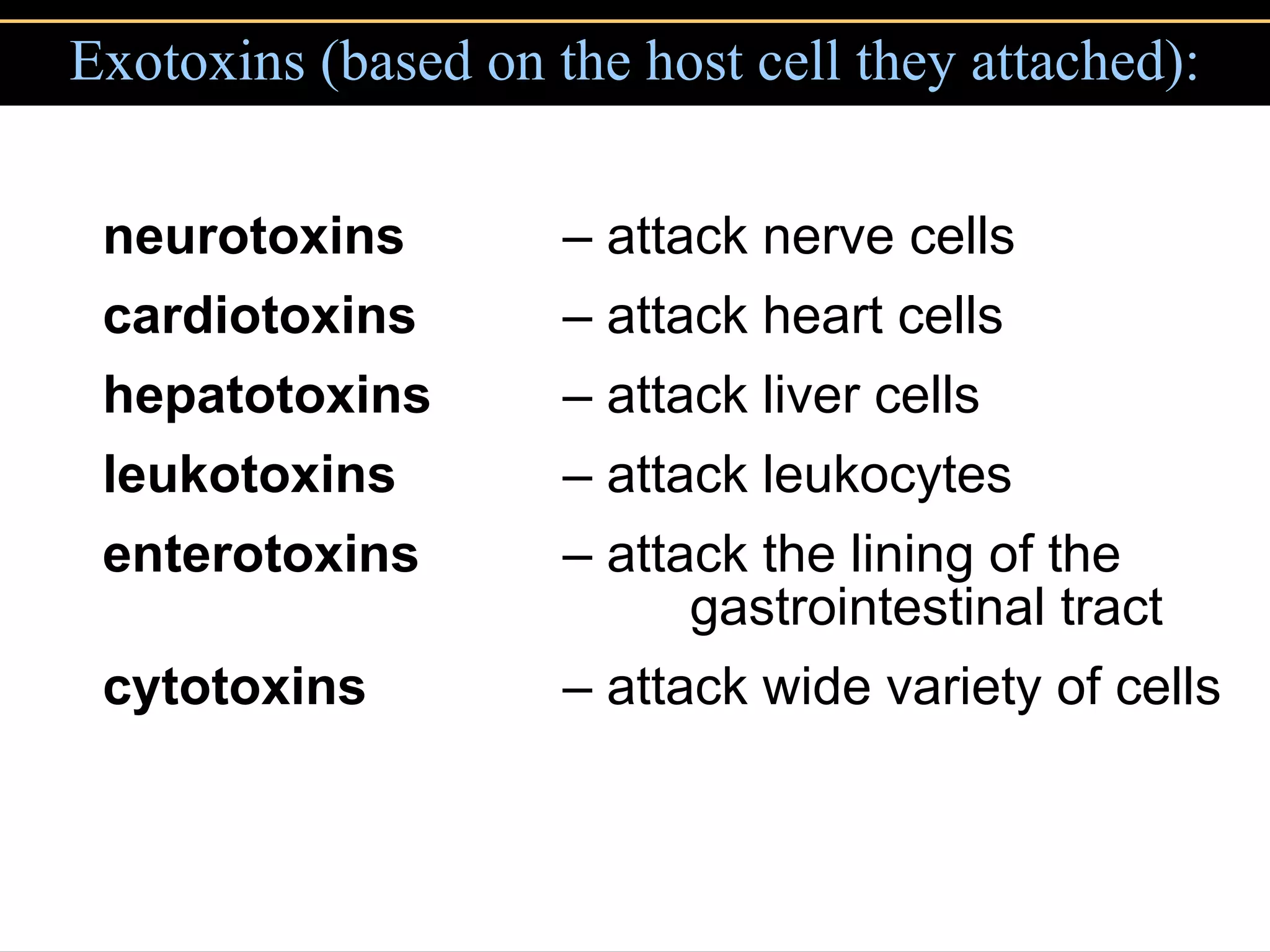
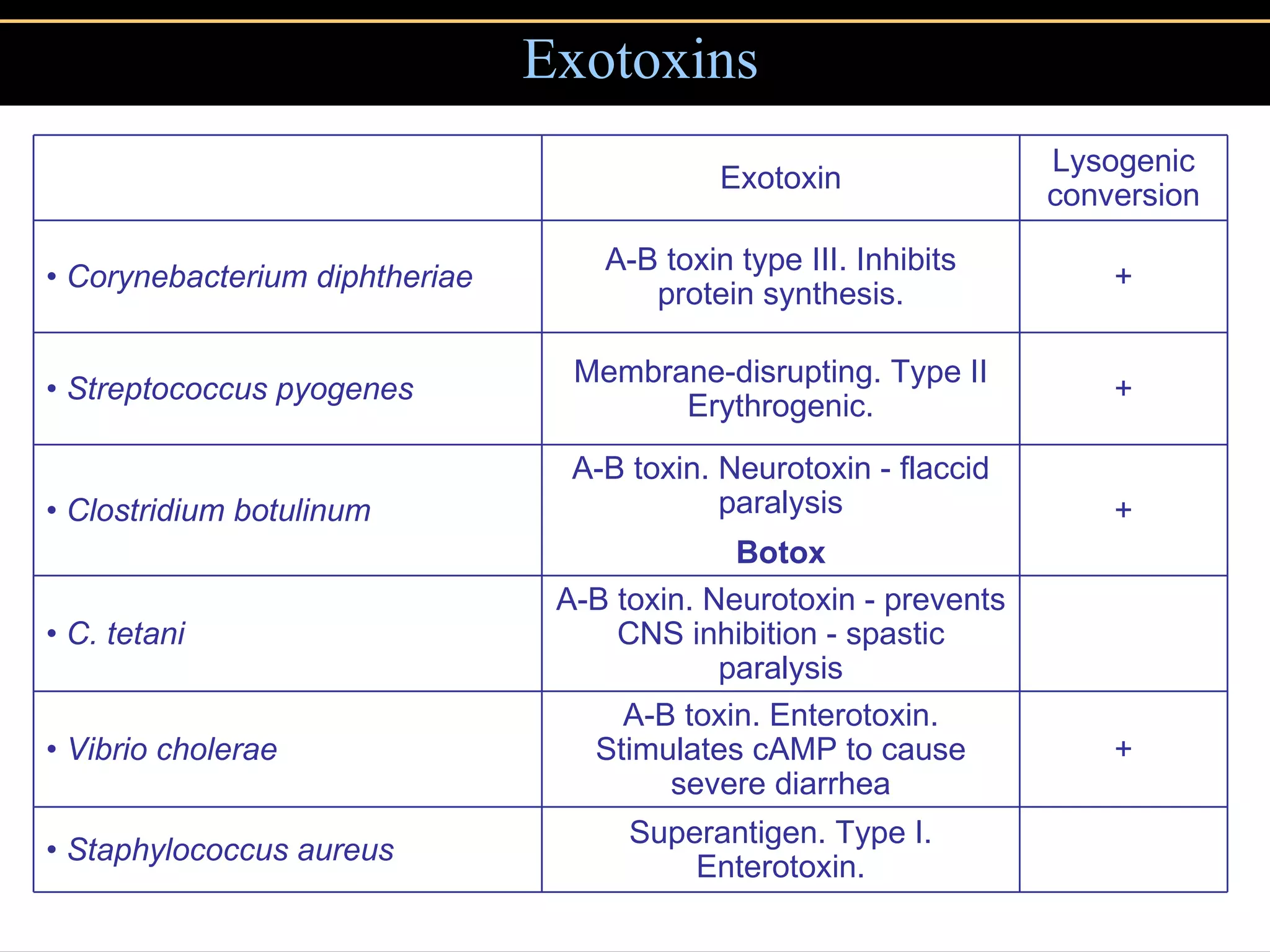
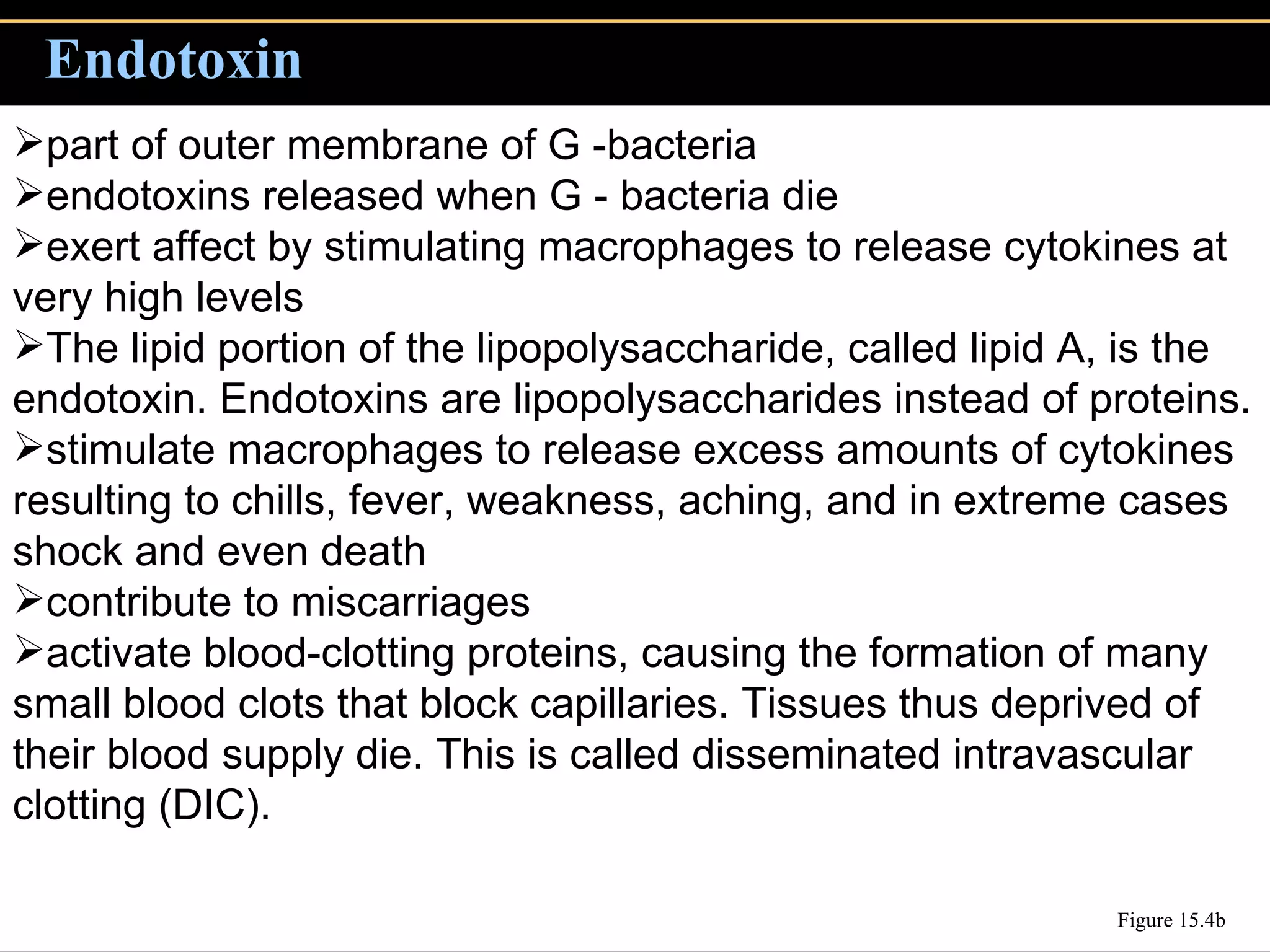
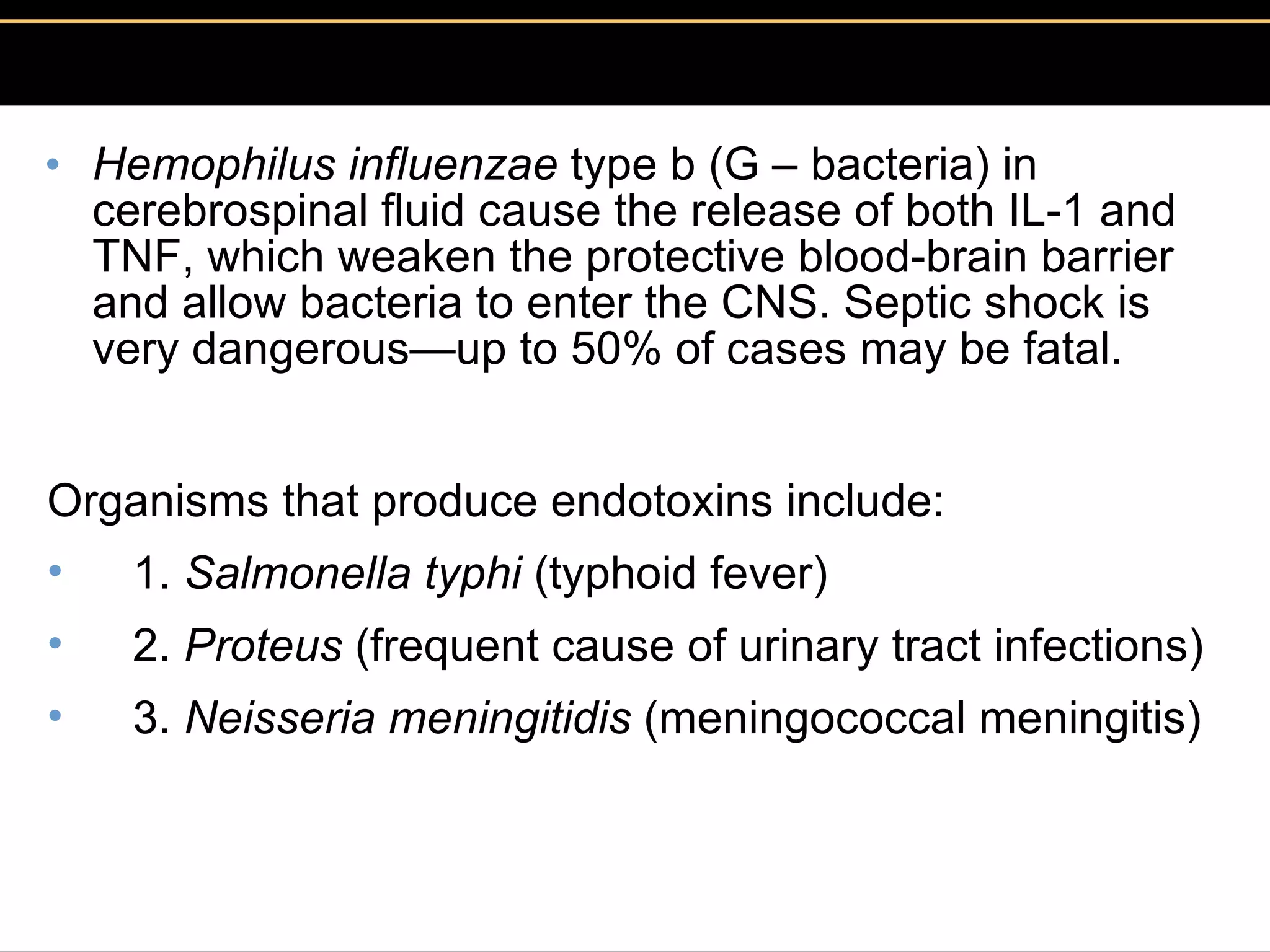
3. Pathogens can directly damage host cells by using their nutrients or producing waste, or cause damage remotely by producing toxins that spread through the bloodstream.