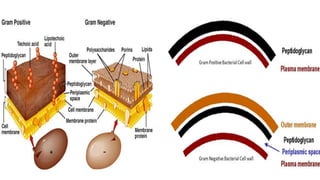
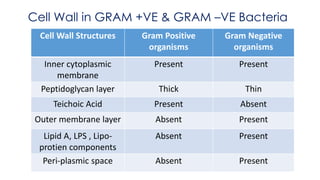
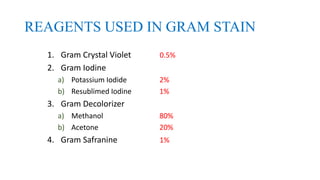
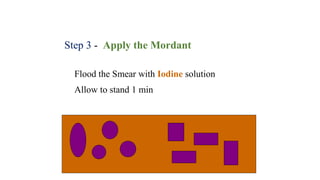
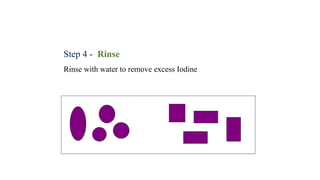
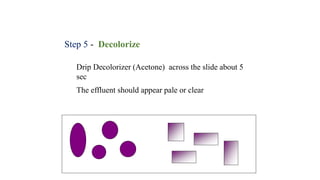
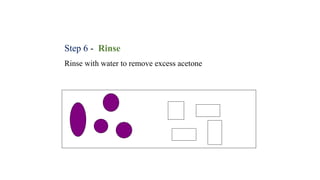
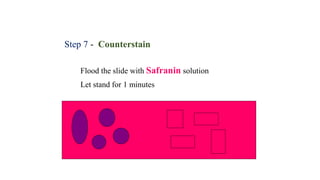
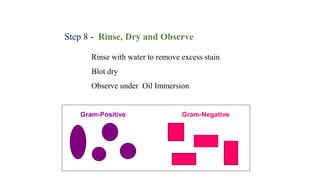
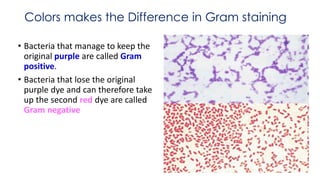
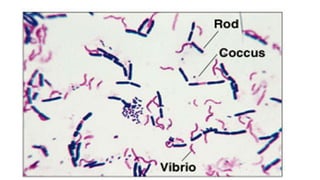
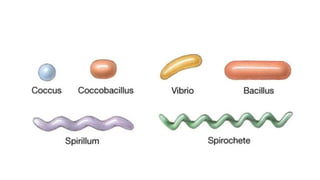
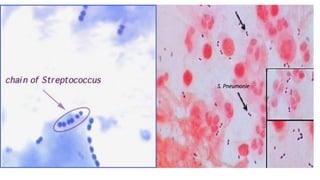
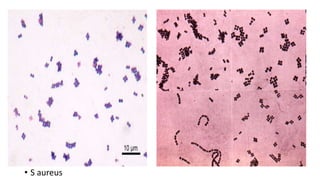
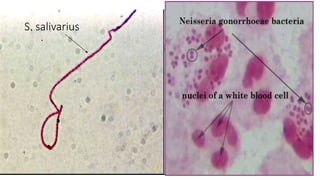
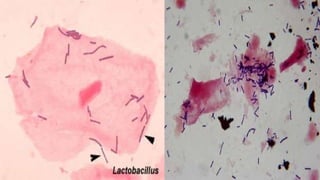
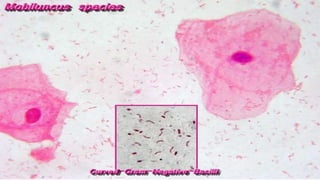
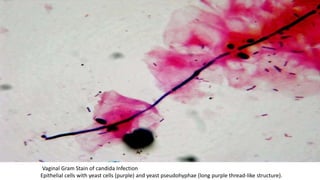
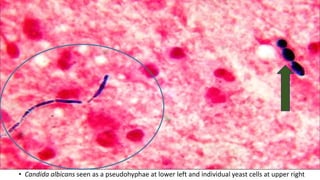
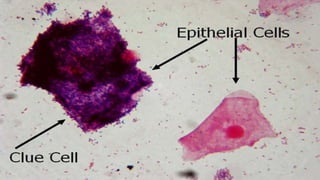
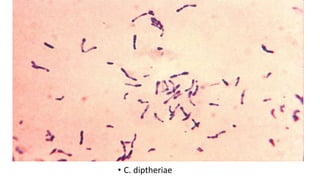
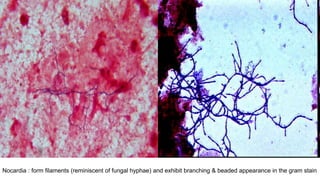
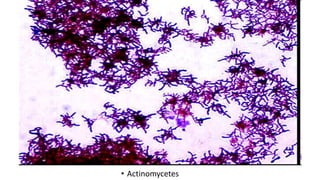
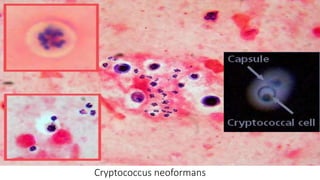
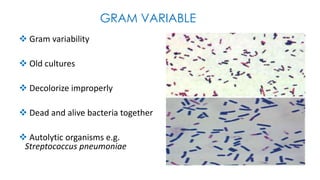
This document provides information about Gram staining, a technique used to classify bacteria. It describes how Gram-positive bacteria retain the crystal violet stain due to their thick peptidoglycan layer, while Gram-negative bacteria do not retain the stain due to their thin peptidoglycan layer and outer membrane. The document outlines the Gram staining procedure and reagents used, and provides examples of morphological characteristics of different bacteria under Gram staining.