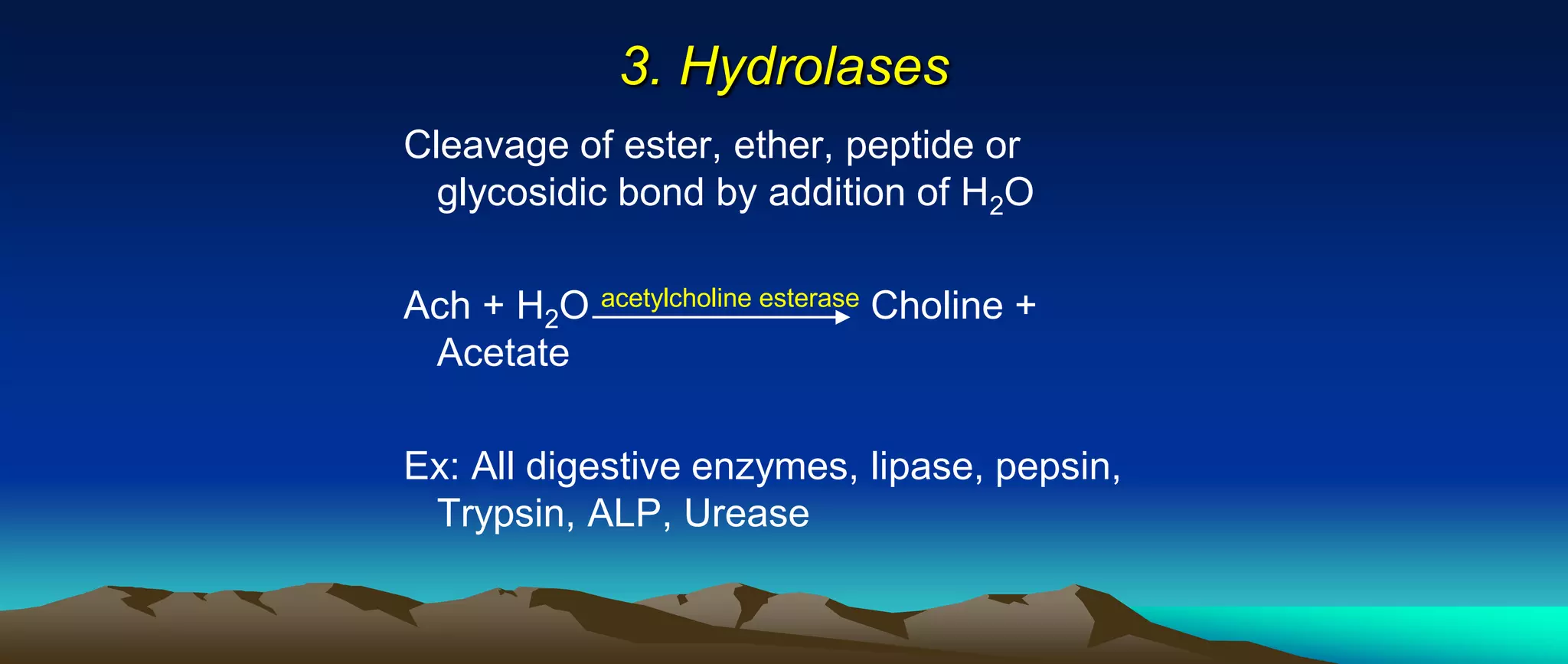
This document provides information about enzymes including their definition, classification, nomenclature, characteristics, and factors that affect their activity. Some key points:
- Enzymes are proteins that act as catalysts for biochemical reactions in living cells and are highly specific. There are six major classes of enzymes based on the type of reaction they catalyze.
- Classification systems such as IUBMB provide unambiguous names for enzymes based on the reaction they catalyze.
- Enzymes have an active site where substrates bind and catalytic residues that participate in reactions. Cofactors such as metals and organic molecules are required for some enzymes to function.
- Factors like concentration of enzyme and substrate, temperature, pH

































![2. Substrate concentration
affecting enzyme activity
E + S ↔ ES ↔ E + P
A
B
C
3 Phases
A. At low substrate
conc.– V α [S]
B. [S] not directly
proportional to V
C. Reaction
independent of [S]](https://image.slidesharecdn.com/enzymologyforb-210719120322/75/Enzymology-for-B-Sc-nursing-students-35-2048.jpg)





![50% molecules of Hexokinase are
saturated even at a lower conc. Of
glucose.
When [S] << Km → reaction is first-
order
When [S] >> Km → reaction is zero-
order](https://image.slidesharecdn.com/enzymologyforb-210719120322/75/Enzymology-for-B-Sc-nursing-students-41-2048.jpg)











![Competitive inhibition
• Inhibitor competes with the substrate for the active
site
• Inhibitor is substrate analogue
• Usually reversible
• ↑ [S] abolishes inhibition
• ↓ velocity of reaction
• ↑ Km
• Vmax unchanged](https://image.slidesharecdn.com/enzymologyforb-210719120322/75/Enzymology-for-B-Sc-nursing-students-53-2048.jpg)


![Non-competitive inhibition
• No competition between substrate and
inhibitor
• Different binding sites
• No structural similarities
• ↑ [S] doesn’t resolve the inhibition
• Usually irreversible
• May be reversible when inhibitor is
removed
• Km value unchanged
• Vmax reduces](https://image.slidesharecdn.com/enzymologyforb-210719120322/75/Enzymology-for-B-Sc-nursing-students-56-2048.jpg)

















