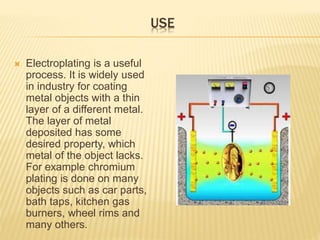
The document discusses electroplating, a process that uses electrical current to form a metal coating on an electrode, altering the surface properties of objects for enhanced characteristics like corrosion resistance and aesthetics. It outlines the procedure of metal deposition, commonly involving a single metallic element, and the use of stop-offs to prevent undesired plating. The document highlights various applications of electroplating in industries, such as chromium plating on automotive parts and household items.