Corrosion is the deterioration of materials due to chemical or electrochemical reaction with the environment. It is an inevitable process that leads to significant economic losses. Corrosion engineering studies corrosion mechanisms and works to prevent or control corrosion economically and safely. Common types of corrosion include galvanic, erosion, crevice, pitting, and microbiologically influenced corrosion. Factors that influence corrosion include the metal properties, environmental conditions like temperature, pH, and presence of ions. Protection methods include material selection, cathodic protection, modifying the environment, metallic coatings, inorganic coatings, and organic coatings.

















![3) The Acid Theory – applicable particularly to
rusting of iron
2Fe + O2 + 4CO2 + 2H2O 2Fe(HCO3)2
2Fe(HCO3)2 + H2O + [O] 2Fe(OH)CO3+2CO2+
2H2O
2Fe(OH)CO3 + 2H2O 2Fe(OH)3 + 2CO2](https://image.slidesharecdn.com/corrosion-130413030451-phpapp02/85/Corrosion-20-320.jpg)
![TYPES OF CORROSION
[I] Galvanic Corrosion (Bimetallic corroson):
E.g. Zinc and copper couple
More reactive Zn Zn2+ + 2e- At anode
(Corrodes)
Less reactive Cu2+ + 2e- Cu At Cathode
(protected)](https://image.slidesharecdn.com/corrosion-130413030451-phpapp02/85/Corrosion-21-320.jpg)



![[II] Erosion Corrosion:
Due to abrading action of flow of gases or
mechanical rubbing action of solids over the
metal surface.
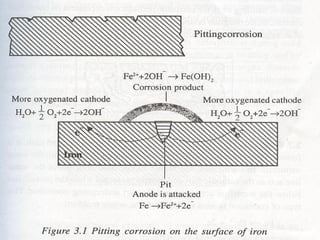
[III] Crevice Corrosion:
Due to cracks in paint coating
[IV] Pitting Corrosion:
Most dangerous form of corrosion as it leads to
sudden failure of material due to formation of
holes.](https://image.slidesharecdn.com/corrosion-130413030451-phpapp02/85/Corrosion-25-320.jpg)


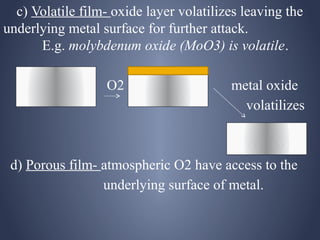
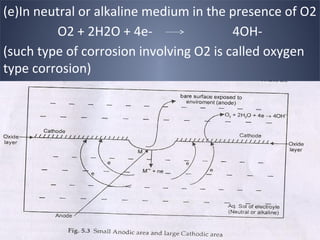
![[IV] Differential aeration Corrosion (Oxygen
Concentration Cell Corrosion)
• One part of the metal is exposed to a different
air/O2 concentration from the rest of the part.
• Portion with lesser O2 = Anode
• Portion with more O2 = Cathode
• e.g. A iron nail inside the wood undergoes
corrosion easily](https://image.slidesharecdn.com/corrosion-130413030451-phpapp02/85/Corrosion-29-320.jpg)
![[V] Waterline Corrosion](https://image.slidesharecdn.com/corrosion-130413030451-phpapp02/85/Corrosion-30-320.jpg)
![[VI] Micro-Biological Corrosion :
Due to metabolic activity of various micro-
organisms
[VII] Stress-Corrosion Cracking
•Metal under stress becomes more anodic and
tend to increase the rate of corrosion.
•The stress can be due to non-uniform
deformation by unequal cooling from high
temperature as in welding](https://image.slidesharecdn.com/corrosion-130413030451-phpapp02/85/Corrosion-31-320.jpg)




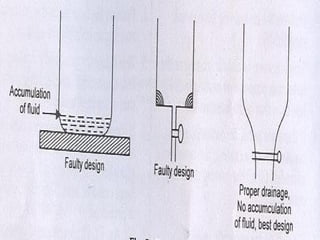
![PROTECTION FROM CORROSION
[I] Design and Material Selection](https://image.slidesharecdn.com/corrosion-130413030451-phpapp02/85/Corrosion-36-320.jpg)

![[II] Cathodic Protection
(i) By appropriate galvanic coupling:](https://image.slidesharecdn.com/corrosion-130413030451-phpapp02/85/Corrosion-40-320.jpg)


![[III] Modifying the Environment
i) Deaeration
ii) Deactivation : addition of chemicals, capable of
combining rapidly with O2 in aqueous solution
iii) Dehumidification: by using alumina or silica gel
iv) Alkaline neutralization
v) Use of inhibitors
a. By forming a layer in between which acts as a
barrier between the material and environment.
b. Or by retarding the anodic or cathodic or both
processes](https://image.slidesharecdn.com/corrosion-130413030451-phpapp02/85/Corrosion-43-320.jpg)


