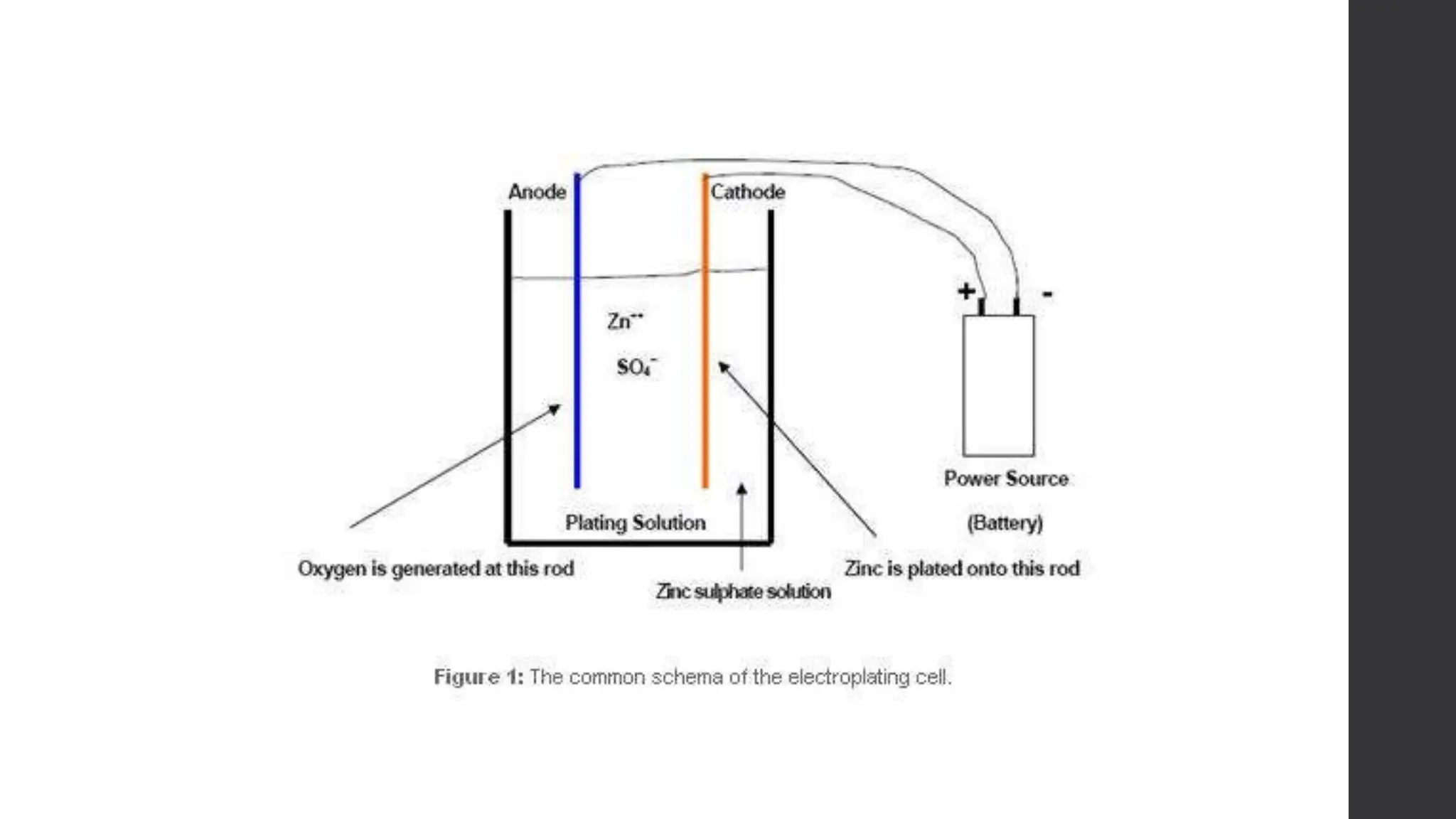
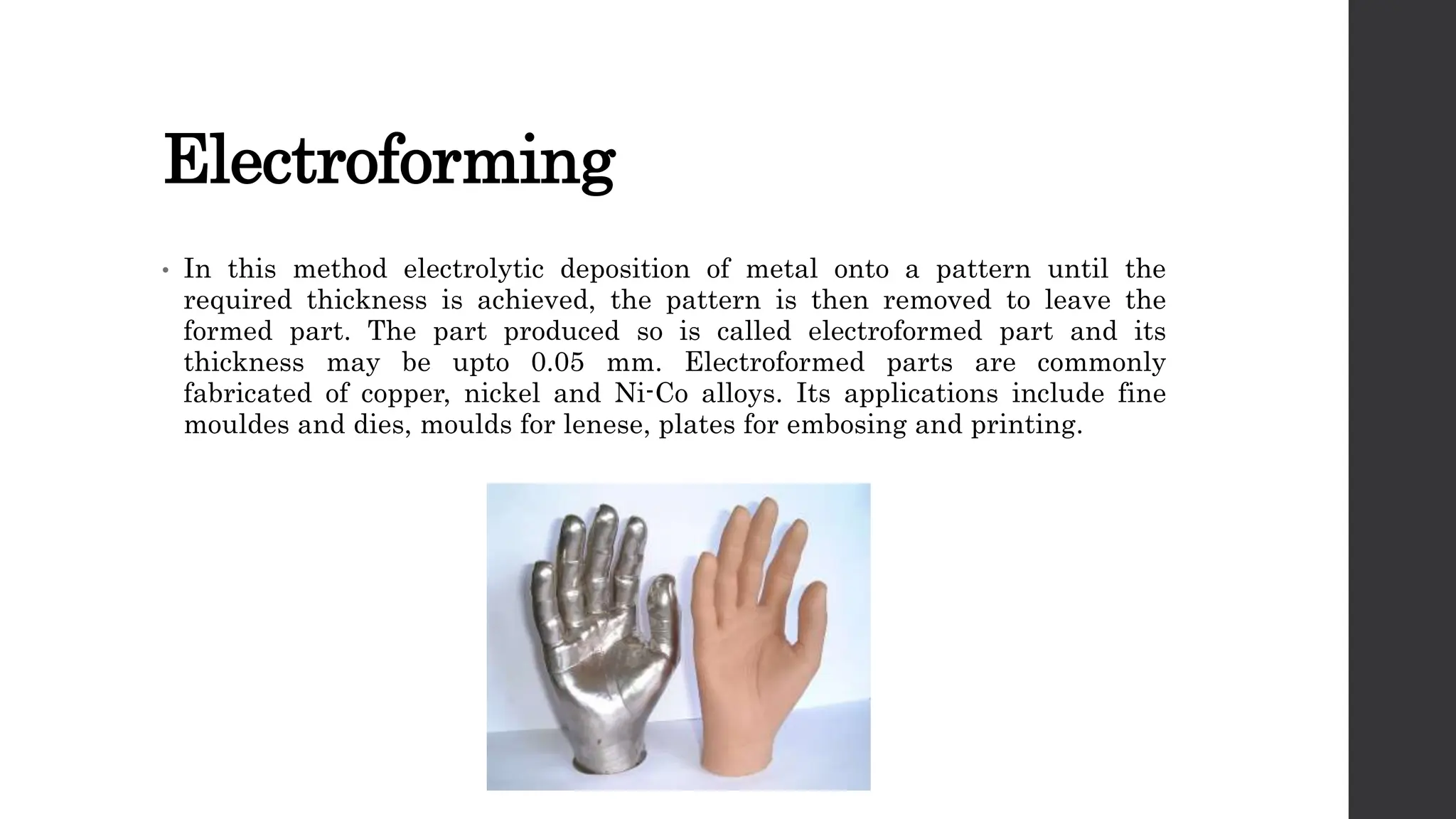
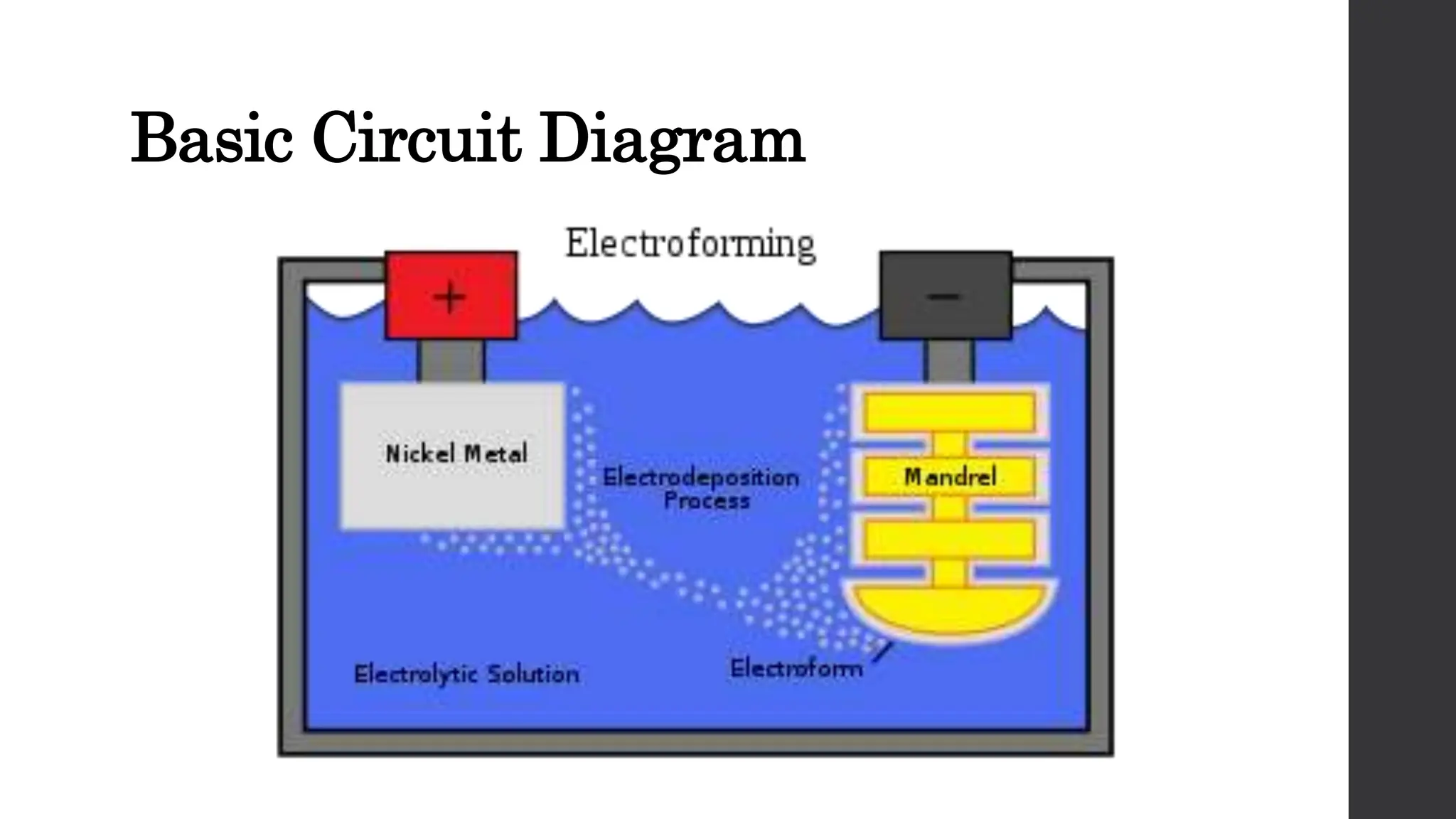
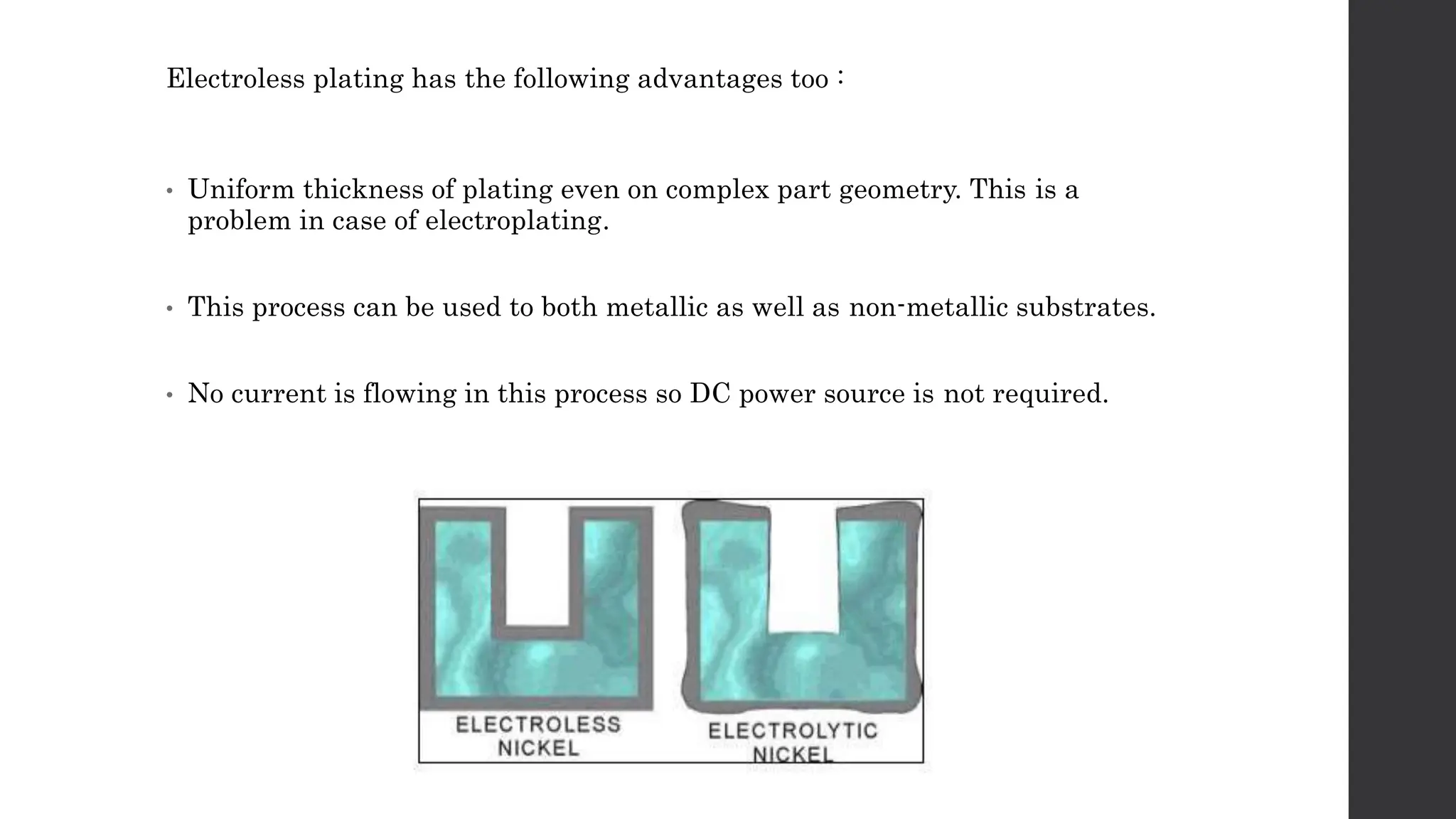
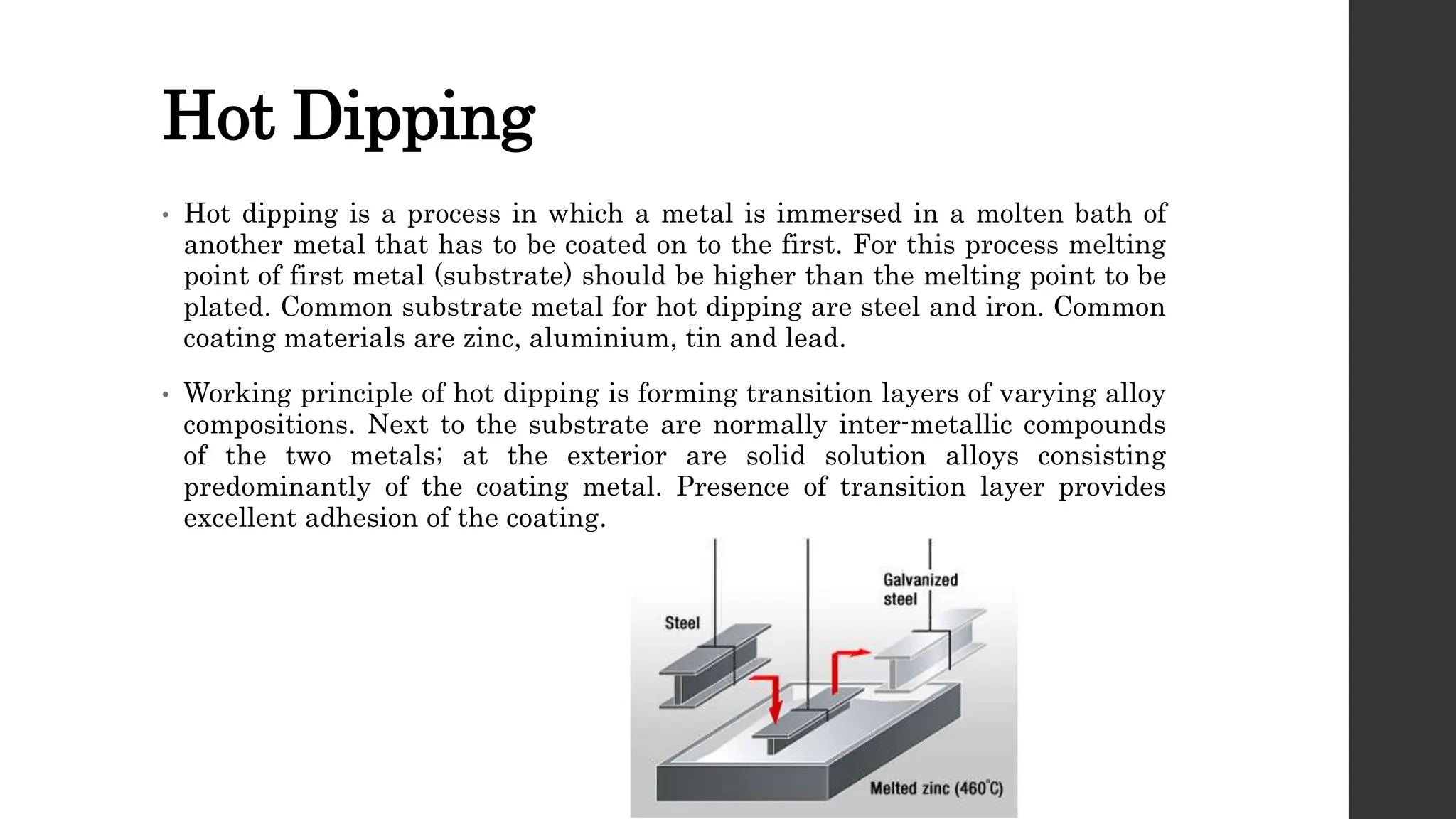
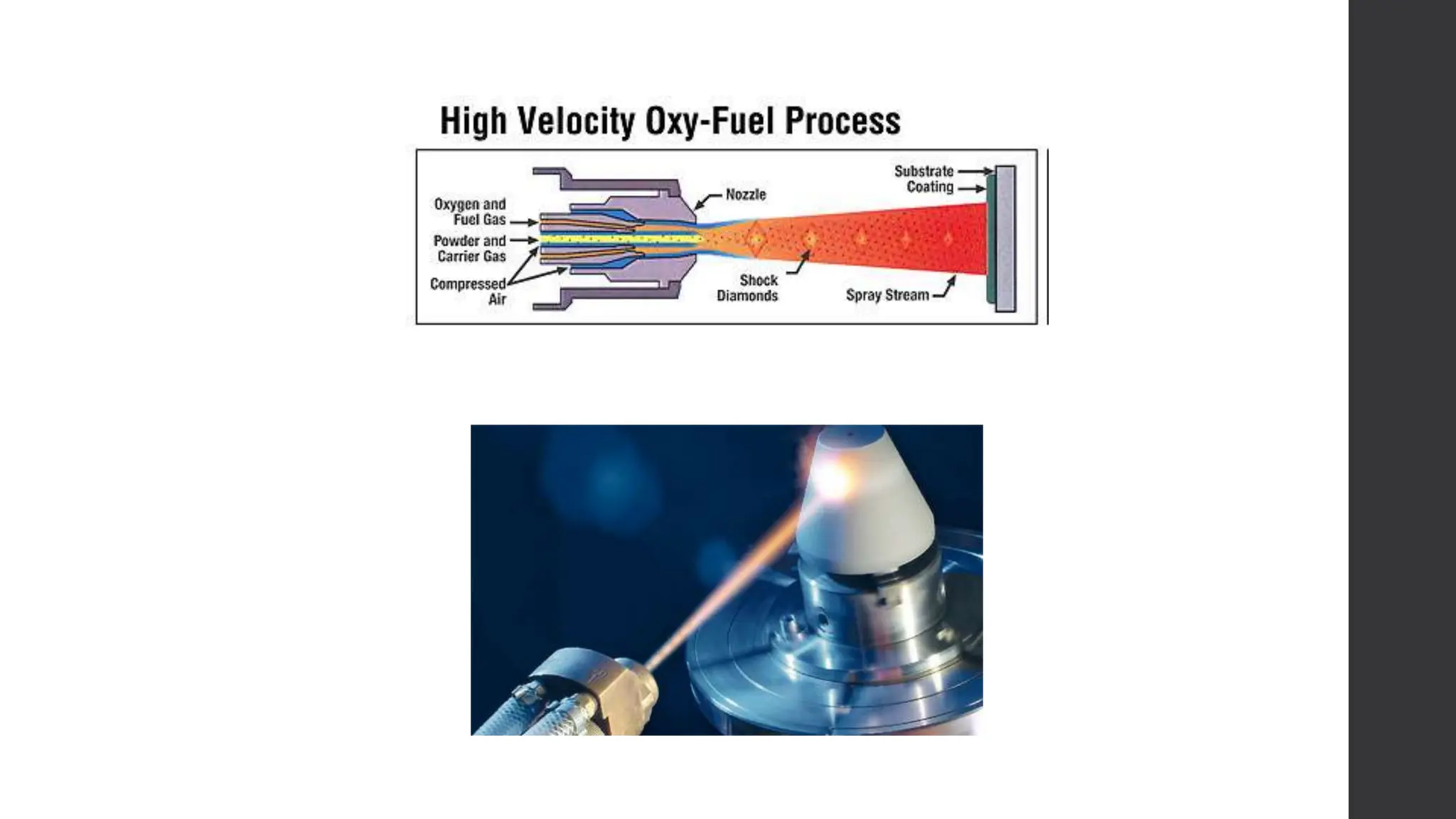
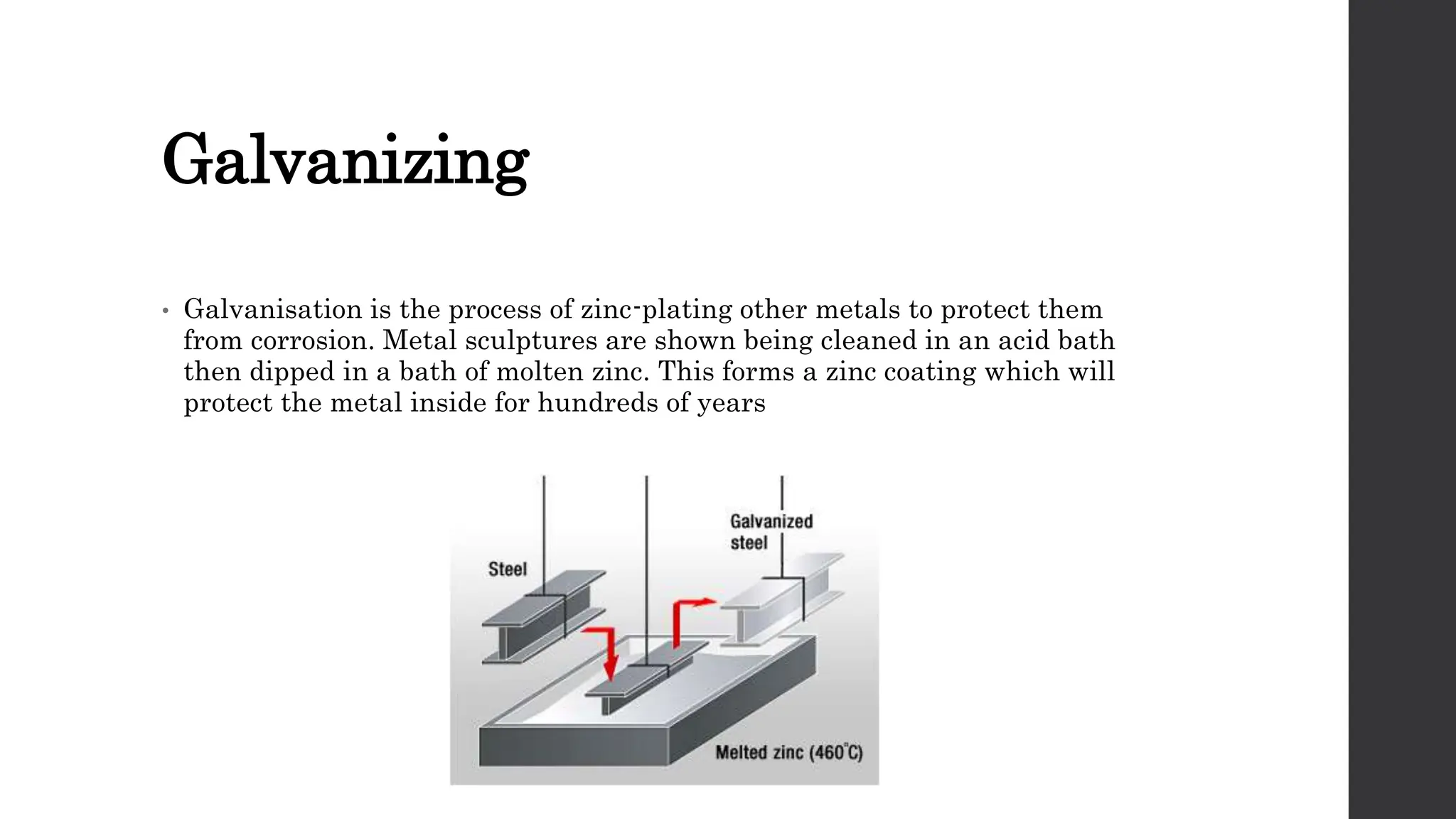
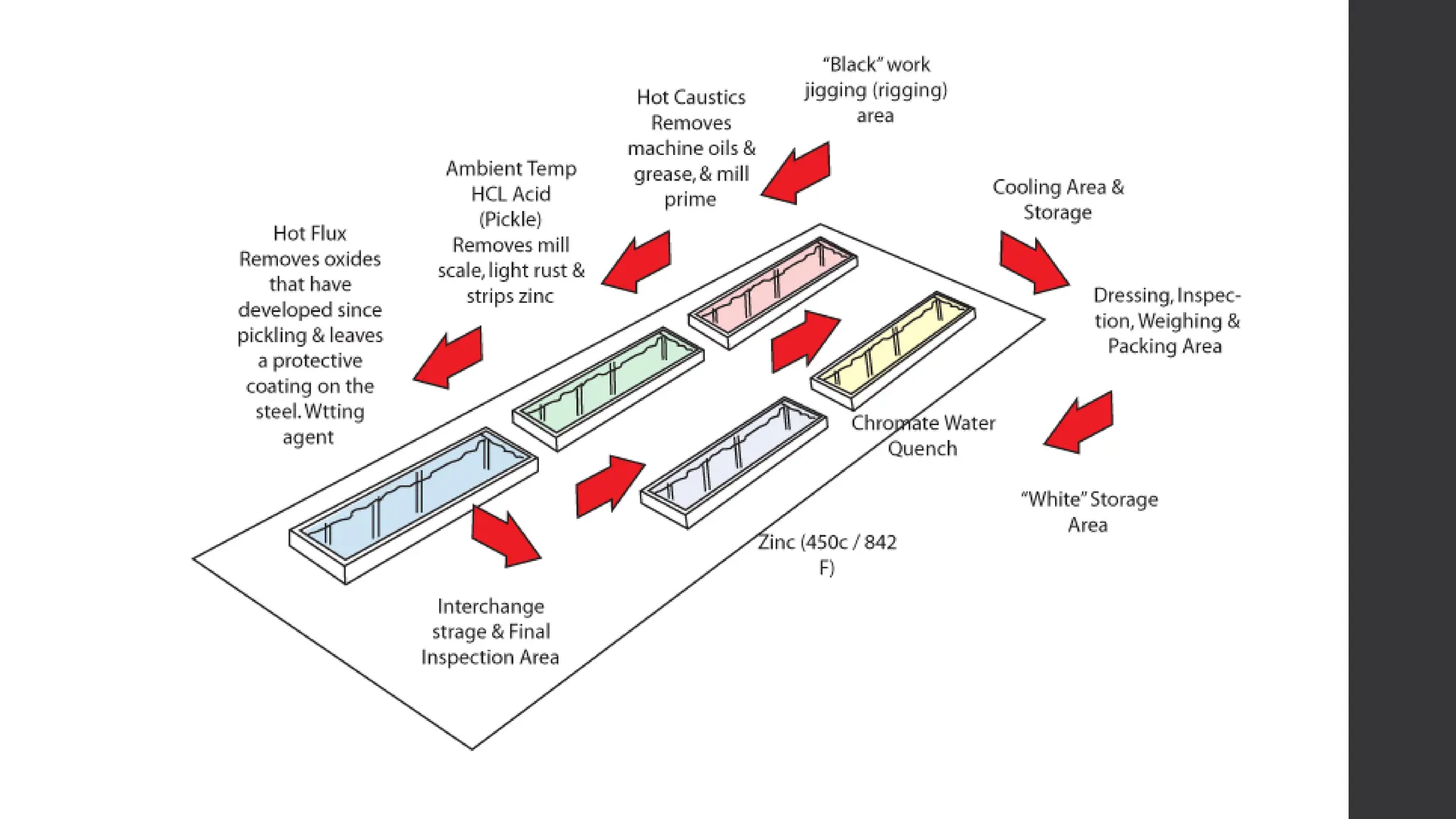
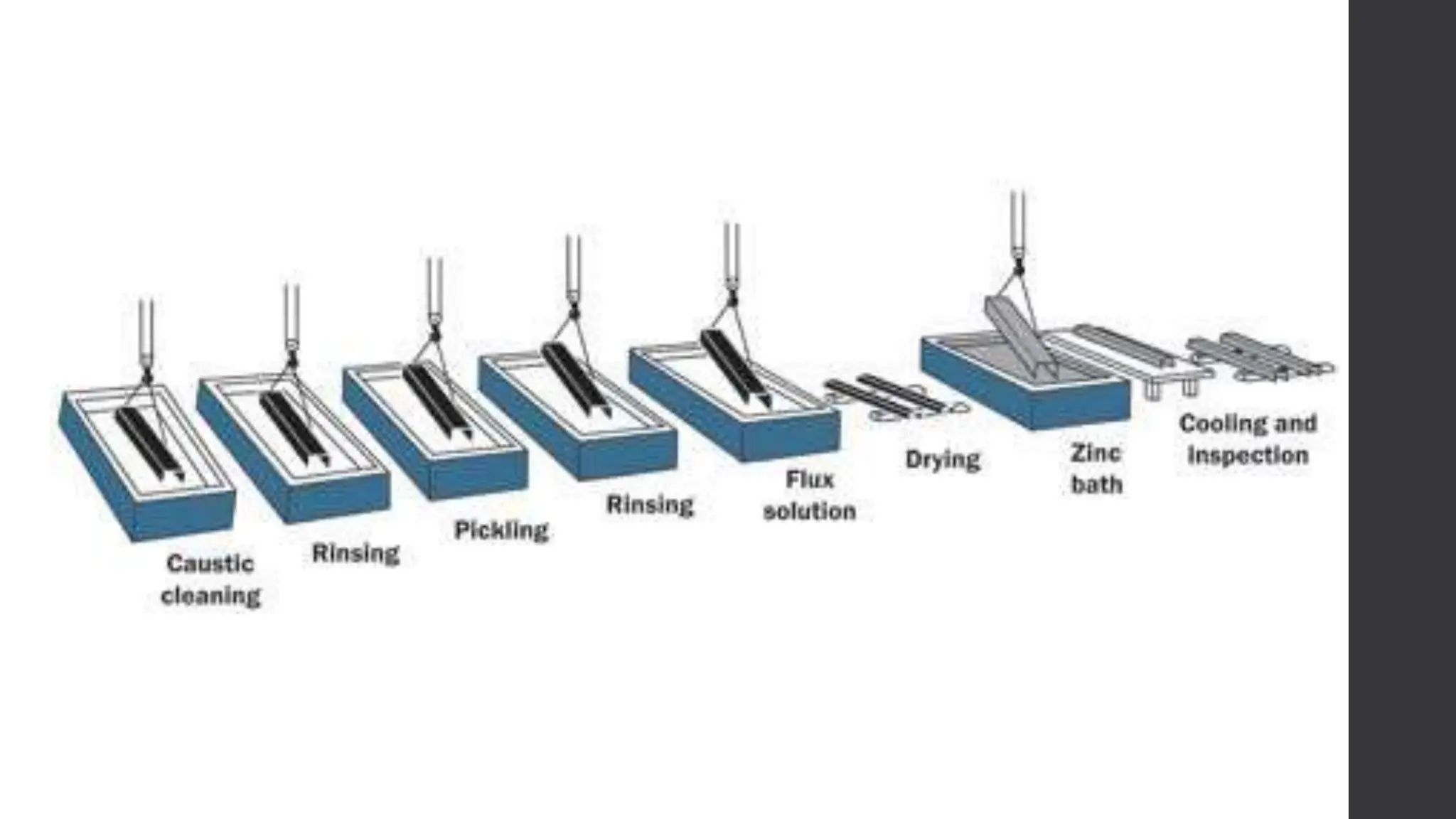
This document provides an overview of surface coating technology. It discusses metallic and non-metallic coatings, comparing their materials and applications. Several coating processes are described, including electroplating, electroless plating, hot dipping, and wire spraying. Electroplating involves depositing metal ions onto a cathode using an electrolytic process. Common metals plated include zinc, nickel, gold, chrome, and tin. Electroless plating is a chemical process that plates without electricity. Hot dipping involves immersing a substrate in molten coating metal.





























![Sherardising
• Sherardizing is a process of galvanization of ferrous metal surfaces, also
called vapour galvanising and dry galvanizing
• This process involves heating the steel parts up to ca. 500°C in a closed
rotating drum that also contains metallic zincdust and possibly an inert
filler, such as sand.[5] At temperatures above 300°C, zinc evaporates and
diffuses into the steel substrate forming diffusion bonded Zn-Fe-phases
• Sherardising is ideal for small parts and parts that require coating of inner
surfaces, such as batches of small items. Part size is only limited by the
drum size. It is reported that pipes up to 6 m in length for the oil industry
are sherardised
• During and shortly after World War I, German 5 Pfennig and 10 Pfennig
coins were sherardised](https://image.slidesharecdn.com/surfacecoatingtechnology-240119161828-0a3a82be/75/Surface-coating-technology-mechanical-engineering-pptx-37-2048.jpg)
![Electroplating Galvanising
• Electrogalvaning is a process in which a layer of zinc is bonded to steel in
order to protect against corrosion. The process involves electroplating,
running a current of electricity through a saline/zinc solution with
a zinc anode and steel conductor
• Zinc electroplating maintains a dominant position among other
electroplating process options, based upon electroplated tonnage per annum.
According to the International Zinc Association, more than 5 million tons are
used yearly for both Hot Dip Galvanizing and Electroplating.[1] ThePlating of
Zinc was developed at the beginning of the 20th century
• The corrosion protection afforded by the electrodeposited zinc layer is
primarily due to the anodic potential dissolution of zinc versus iron (the
substrate in most cases). Zinc acts as a sacrificial anode for protecting the
iron (steel).
• Steel is preserved from corrosion by cathodic protection electroplated zinc
articles may receive a topcoat to further enhance corrosion protection and
friction performance](https://image.slidesharecdn.com/surfacecoatingtechnology-240119161828-0a3a82be/75/Surface-coating-technology-mechanical-engineering-pptx-39-2048.jpg)