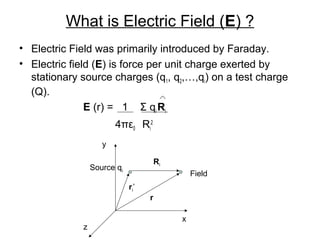
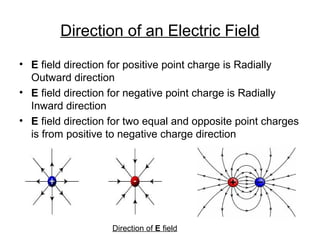
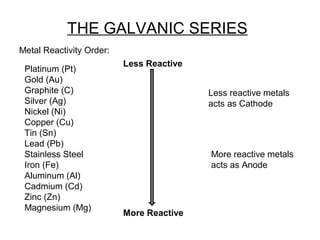
The document discusses electric fields, including their definition, direction, and properties, as well as their applications in electroplating. It explains the relationship between electric field and electric potential using examples and outlines the process of electro-deposition. Additionally, it highlights the various industrial uses of electroplating, such as enhancing appearance, preventing corrosion, and improving surface properties.