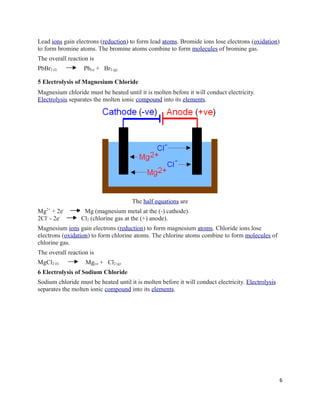
The document outlines the application of electrochemistry in the extraction and purification of metals such as potassium, sodium, lithium, calcium, magnesium, aluminium, copper, and zinc. It discusses various methods for metal extraction based on their reactivity, emphasizing that more reactive metals are typically extracted via electrolysis, while less reactive metals can be obtained through cheaper methods like reduction with carbon. Additionally, the document addresses the advantages and disadvantages of electrolysis, including its efficiency and cost implications.