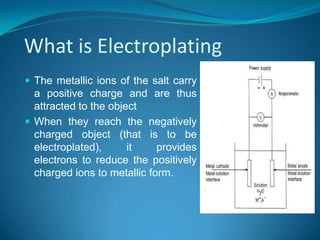
Electroplating is a process where a metal coating is produced on a surface through the action of an electric current in an electrolyte solution. Key factors that influence the quality of electroplating include current density, cathode efficiency, agitation of the plating bath, bath composition/concentration, water quality, and presence of impurities. Hydrogen embrittlement can occur during electroplating and negatively impact the metal's fatigue and mechanical properties.