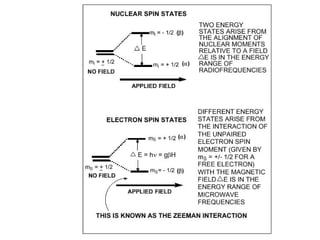
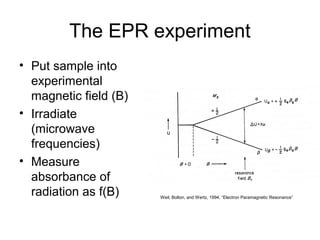
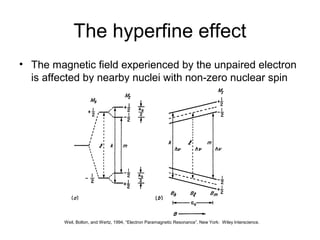
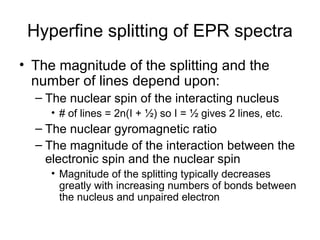
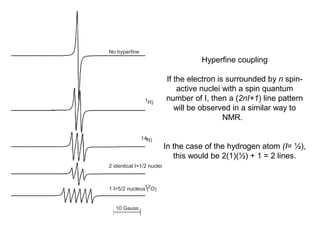
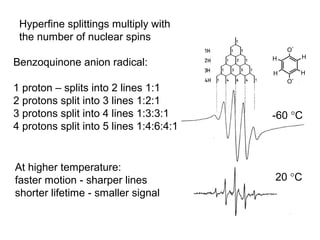
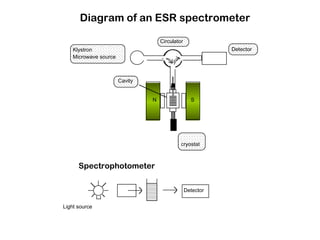
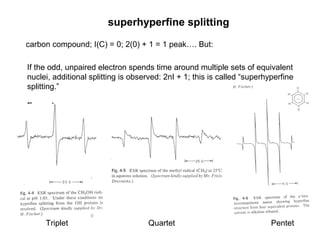
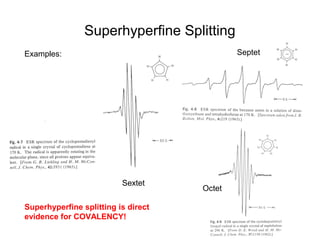
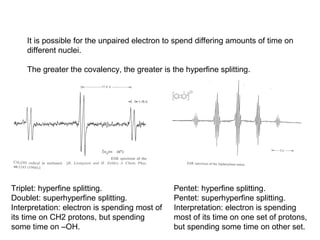
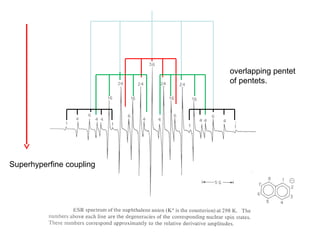
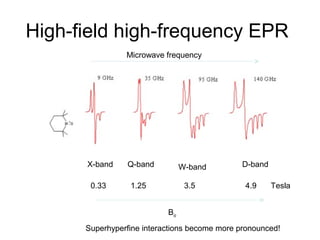
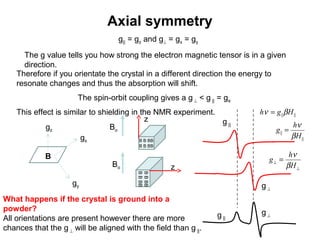
Electron spin resonance (ESR) spectroscopy detects transitions between spin energy levels of unpaired electrons using microwave radiation. When an unpaired electron is near a nucleus with non-zero spin, the electron experiences a magnetic field from the nucleus that splits the ESR signal into multiple lines based on the nuclear spin. This splitting is called hyperfine coupling and provides information about electronic structure. Superhyperfine splitting occurs when the electron interacts with multiple equivalent nuclei and results in even finer splitting patterns. Anisotropic interactions like the g-tensor can also be observed in ESR and provide information about electronic environments.
![Prushan Example
SS
N N
OO
B
FF
Cu
[Cu(Thyclops)]+
+
77 K Cryogenic ESR Spectrum of [Cu(Thyclops)]ClO4
in MeOH
Prushan, M. J.; Addison, A. W.; Butcher, R. J.; Thompson, L. K. “Copper(II) Complex Tetradentate Thioether-Oxime Ligands” Inorganica Chimica
Acta, 358, 3449-3456 (2005).](https://image.slidesharecdn.com/esr-170922021718/85/electron-spin-resonance-spectroscopy-EPR-ESR-13-320.jpg)
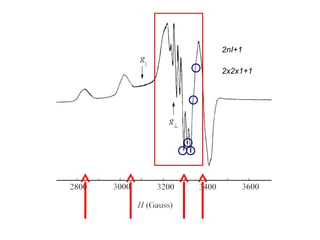
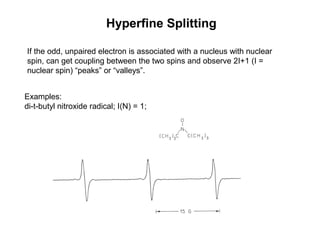
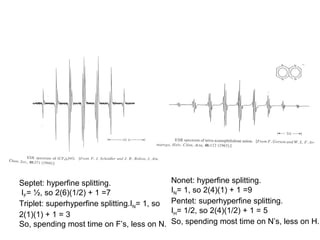
![vanadyl [V=O]2+
complex; I (V) = 7/2; 2(7/2) + 1 = 8 peaks
Hyperfine Splitting](https://image.slidesharecdn.com/esr-170922021718/85/electron-spin-resonance-spectroscopy-EPR-ESR-17-320.jpg)

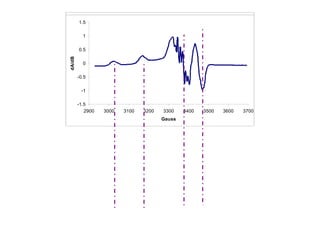
![ESR spectra of [Cu(MeTtoxBF2)]BF4 in
1:10 BuOH–DMF.
(a) Room temperature (295 K) fluid
spectrum (9.464 GHz). (b) 77 K cryogenic
glass spectrum (9.147 GHz).
Prushan, M. J.; Addison, A. W.*; Butcher, R. J.; "Pentadentate
Thioether Oxime Macrocyclic and Quasi-Macrocyclic Complexes of
Copper(II) and Nickel(II)" Inorganica Chimica Acta, 300-302, 992-1003
(2000).](https://image.slidesharecdn.com/esr-170922021718/85/electron-spin-resonance-spectroscopy-EPR-ESR-27-320.jpg)