Embed presentation
Downloaded 45 times

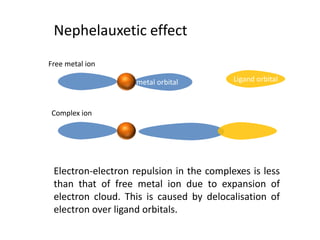
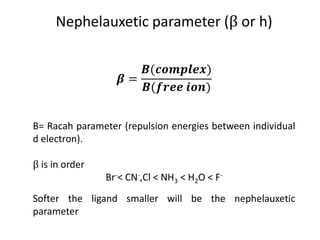
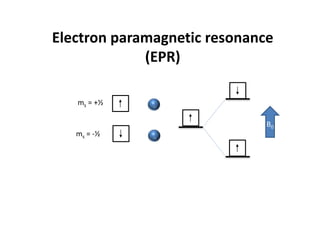
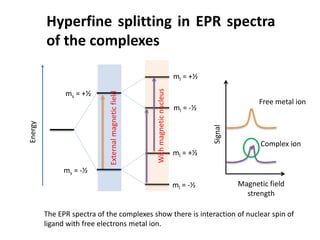

This document discusses evidence for covalent bonding in metal complexes. It explains that electron-electron repulsion is less in complexes than free metal ions due to delocalization of electrons over ligand orbitals. This is known as the nephelauxetic effect. The nephelauxetic parameter (β) quantifies this effect, with softer ligands having a smaller β value. Electron paramagnetic resonance (EPR) spectroscopy provides further evidence, as the spectra of complexes show interaction between the ligand nuclear spin and electrons of the metal ion.





