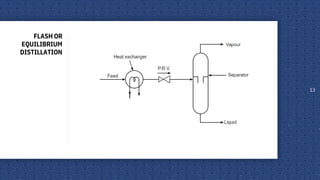
The document discusses distillation as a unit operation for separating liquid mixtures using thermal energy, governed by principles such as Raoult’s Law and Dalton’s Law. It explains various distillation methods including simple, flash, and rectification processes, highlighting the roles of vapour-liquid equilibrium and reflux ratios. Applications of distillation span from separating volatile oils and drugs to purifying organic solvents and refining petroleum products.