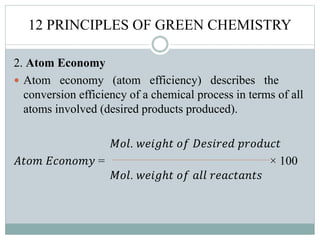
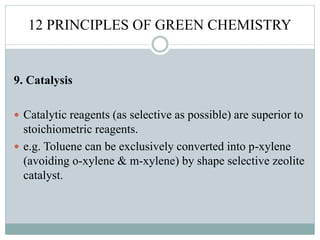
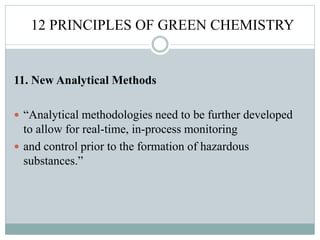
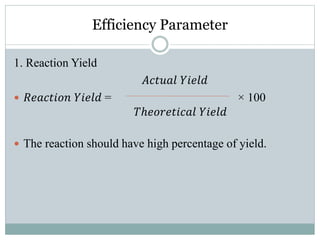
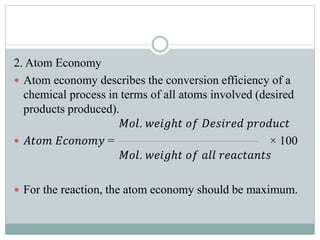
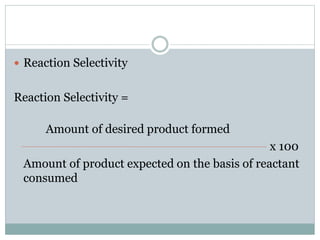
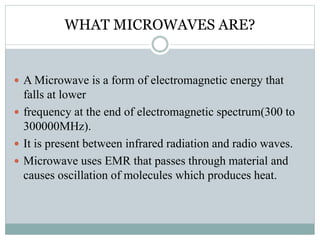
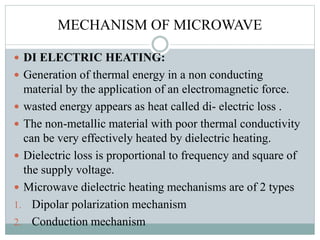
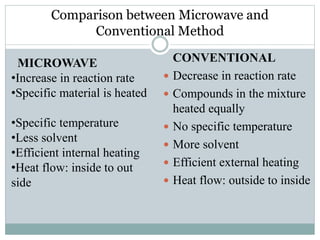
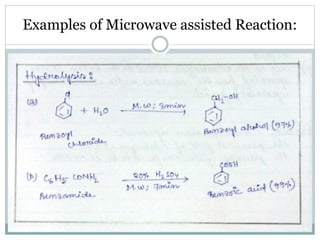
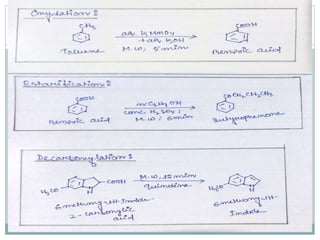
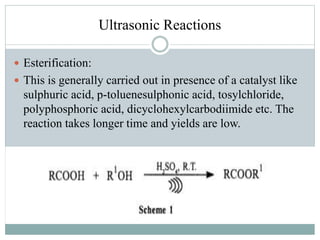
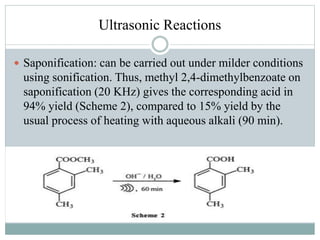
This document provides an overview of green chemistry presented by Jon Jyoti Sahariah. It defines green chemistry and discusses the 12 principles of green chemistry established by Anastas and Warner. These principles guide chemists to design chemical products and processes that reduce risk. The document also discusses green chemistry approaches like using catalysts, renewable resources, and safer solvents. It provides examples of microwave-assisted reactions and ultrasound-mediated reactions as tools of green chemistry that can increase reaction rates and yields.