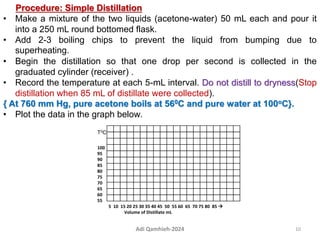
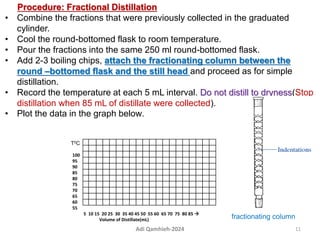
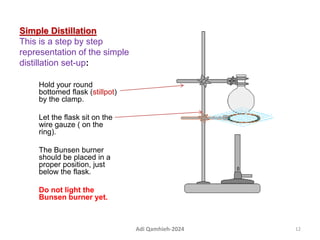
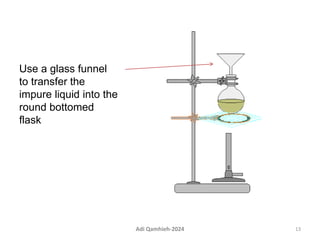
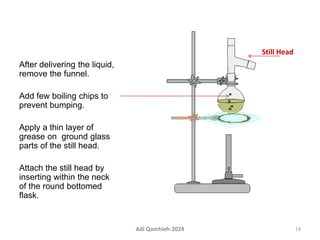
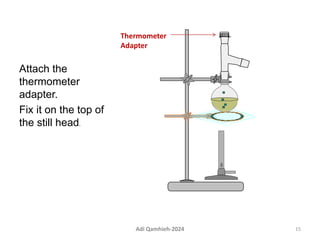
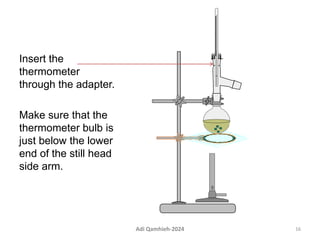
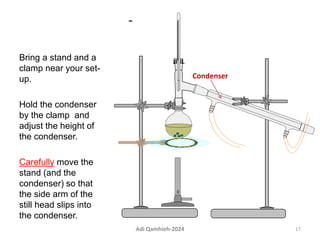
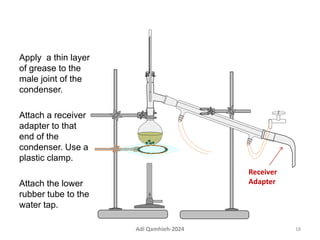
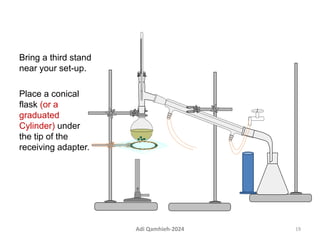
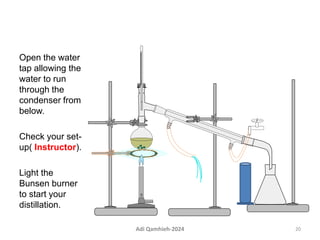
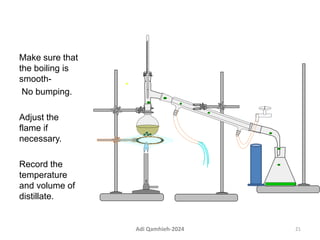
The document describes procedures for simple and fractional distillation. Simple distillation allows separation of compounds with a boiling point difference of over 40-50°C, while fractional distillation using a fractionating column can separate compounds that boil within 50°C of each other. The document outlines the setup and procedure for simple distillation of an acetone-water mixture, including recording temperature and volume collected. Fractional distillation of the same mixture is then described using the fractionating column to achieve better separation.