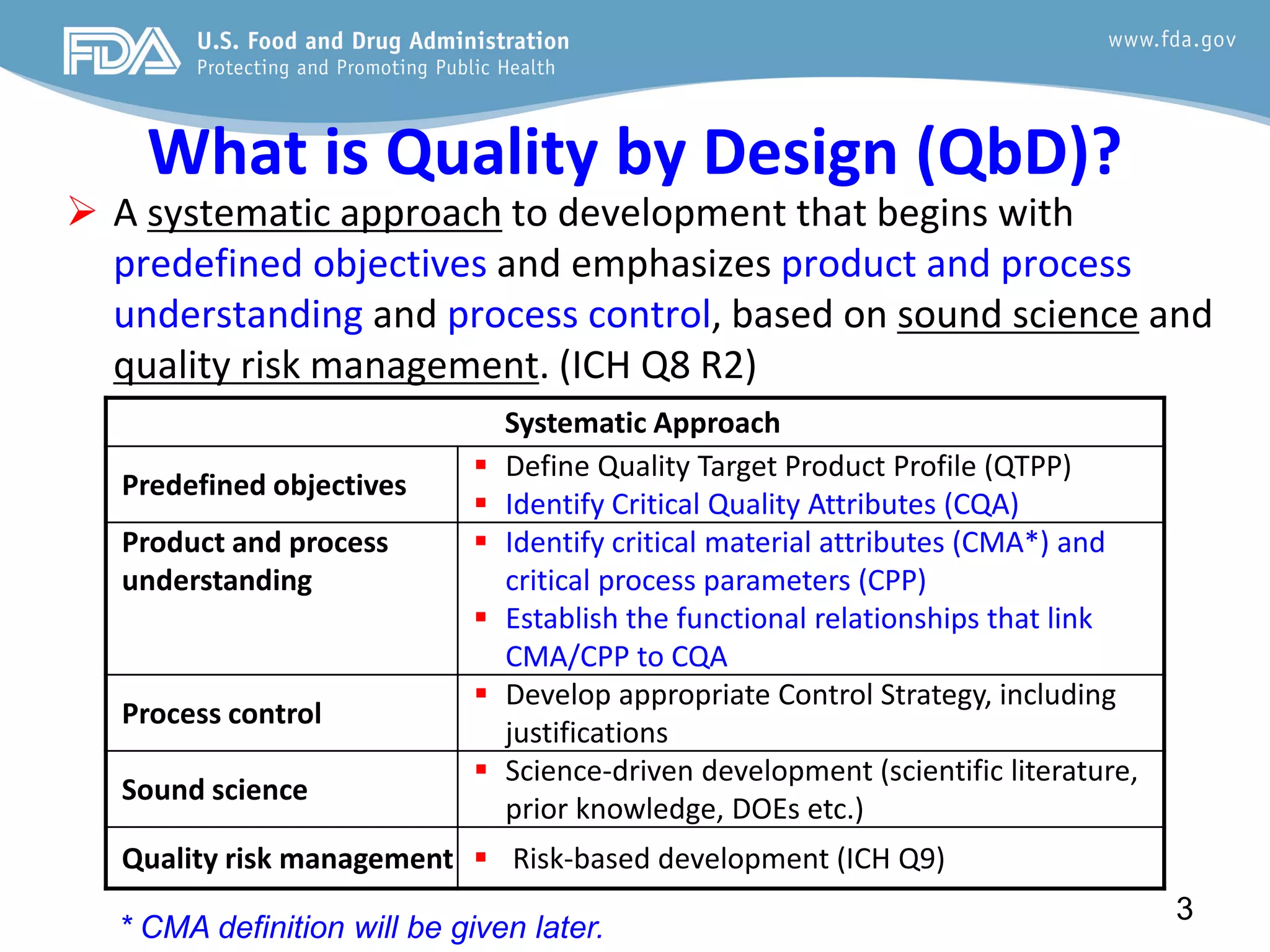
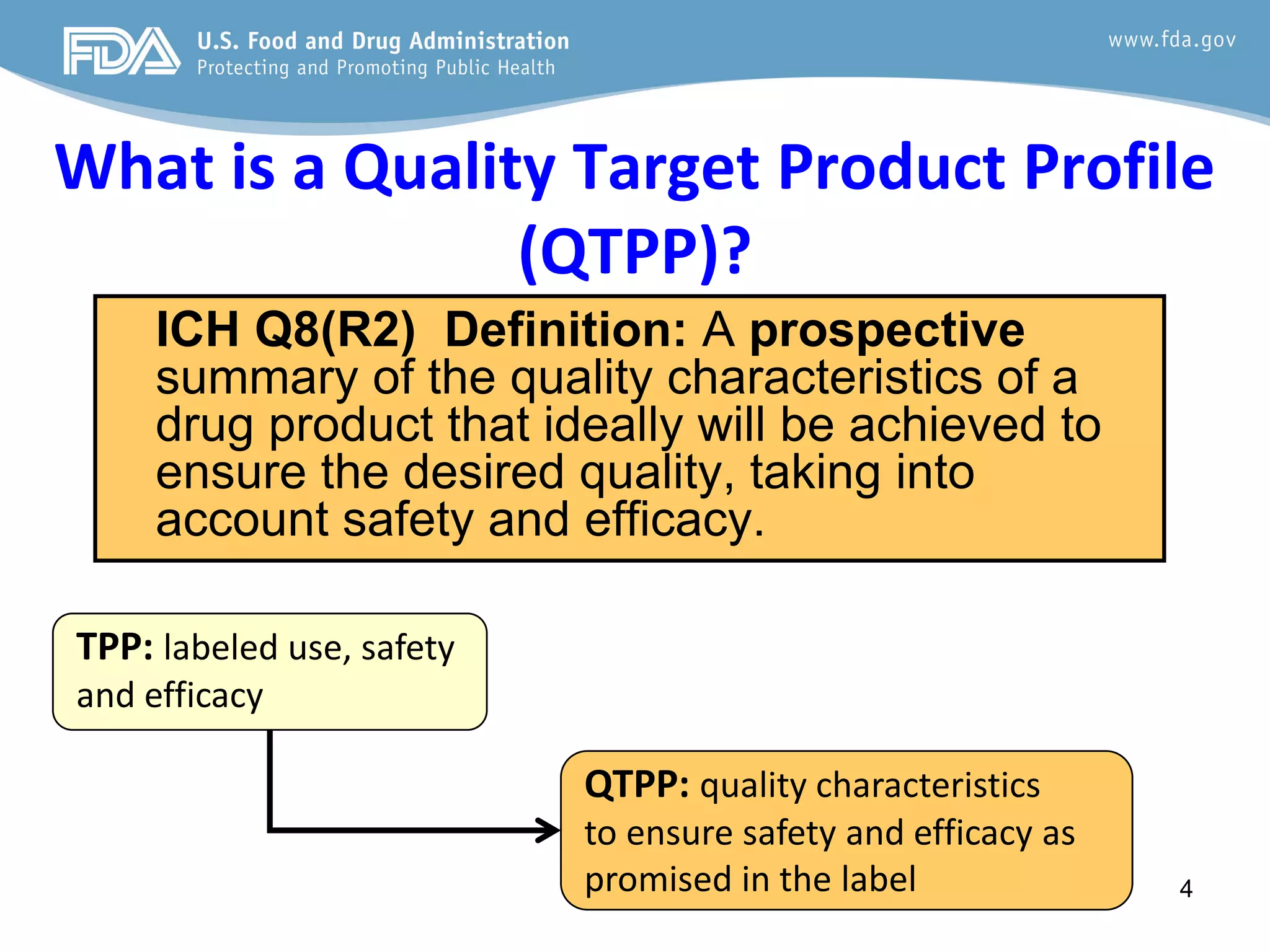
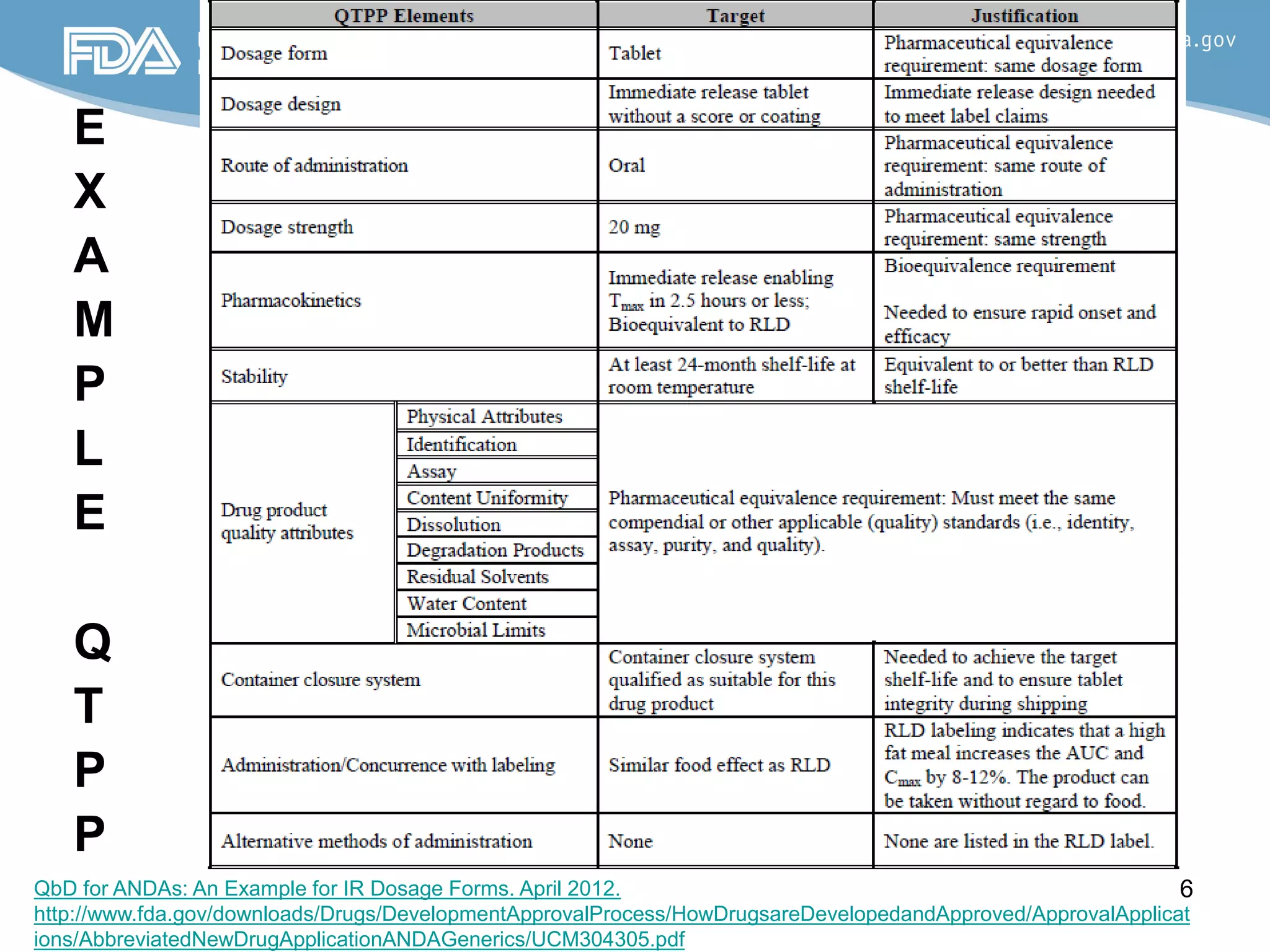
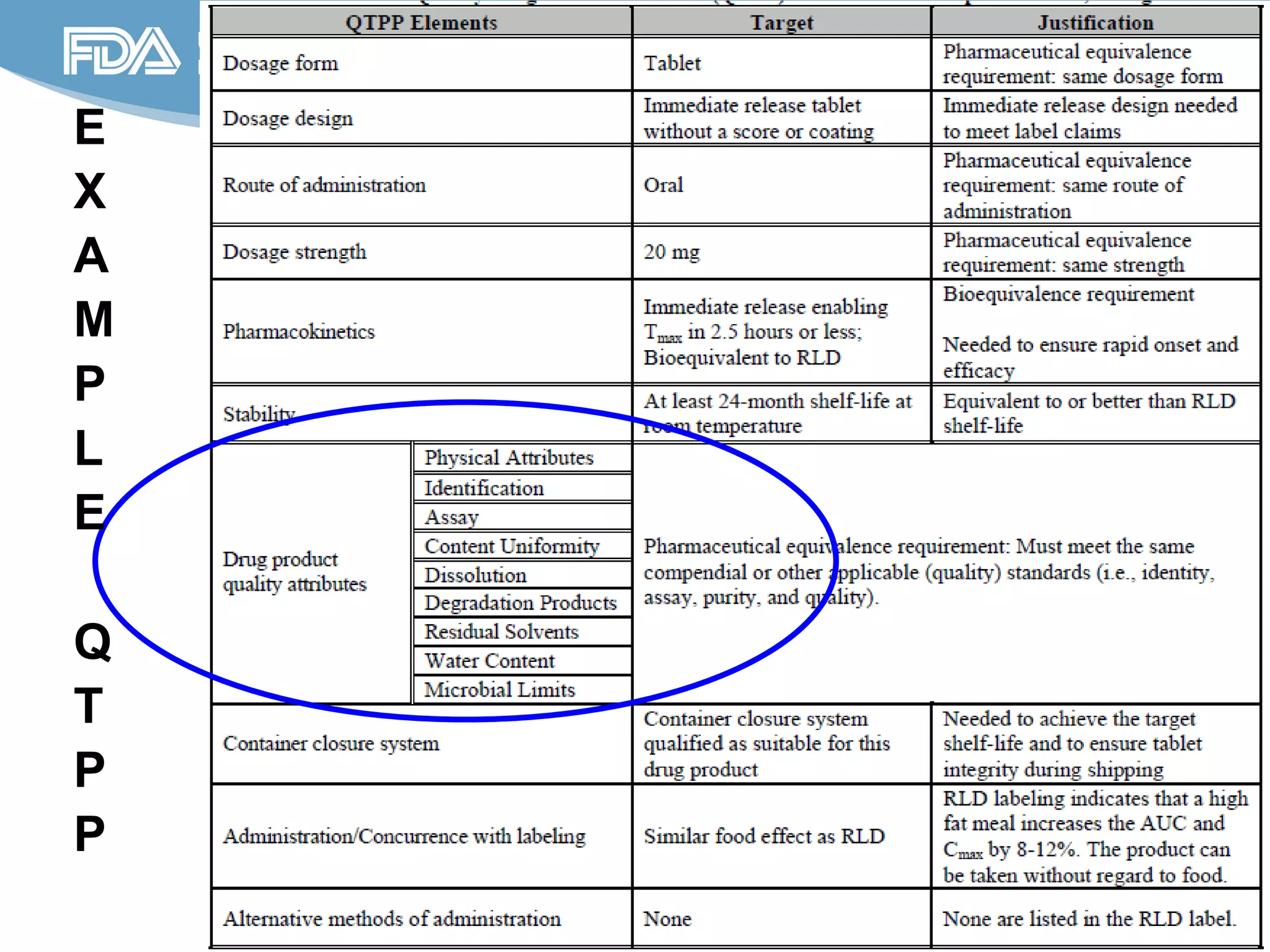
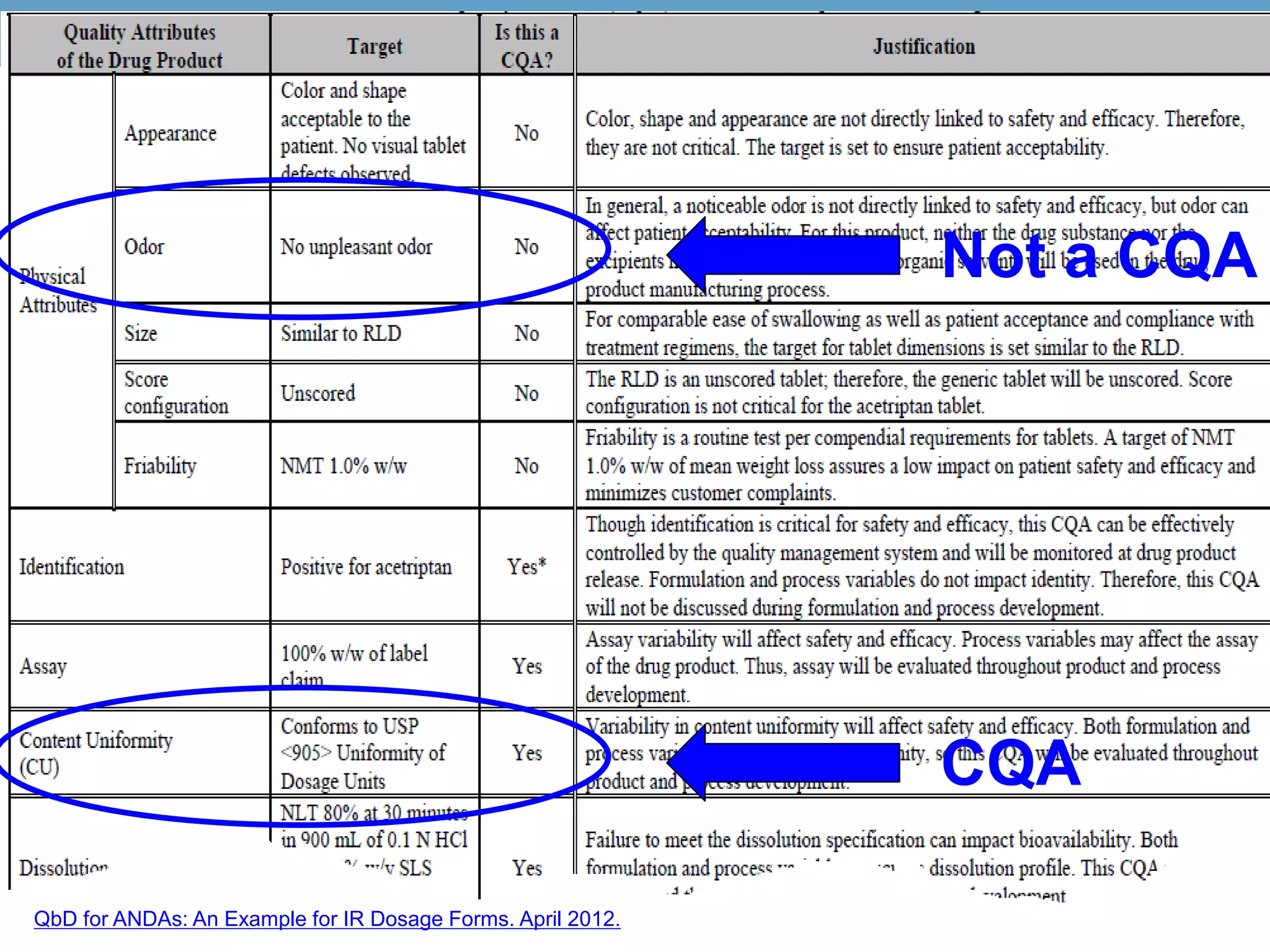
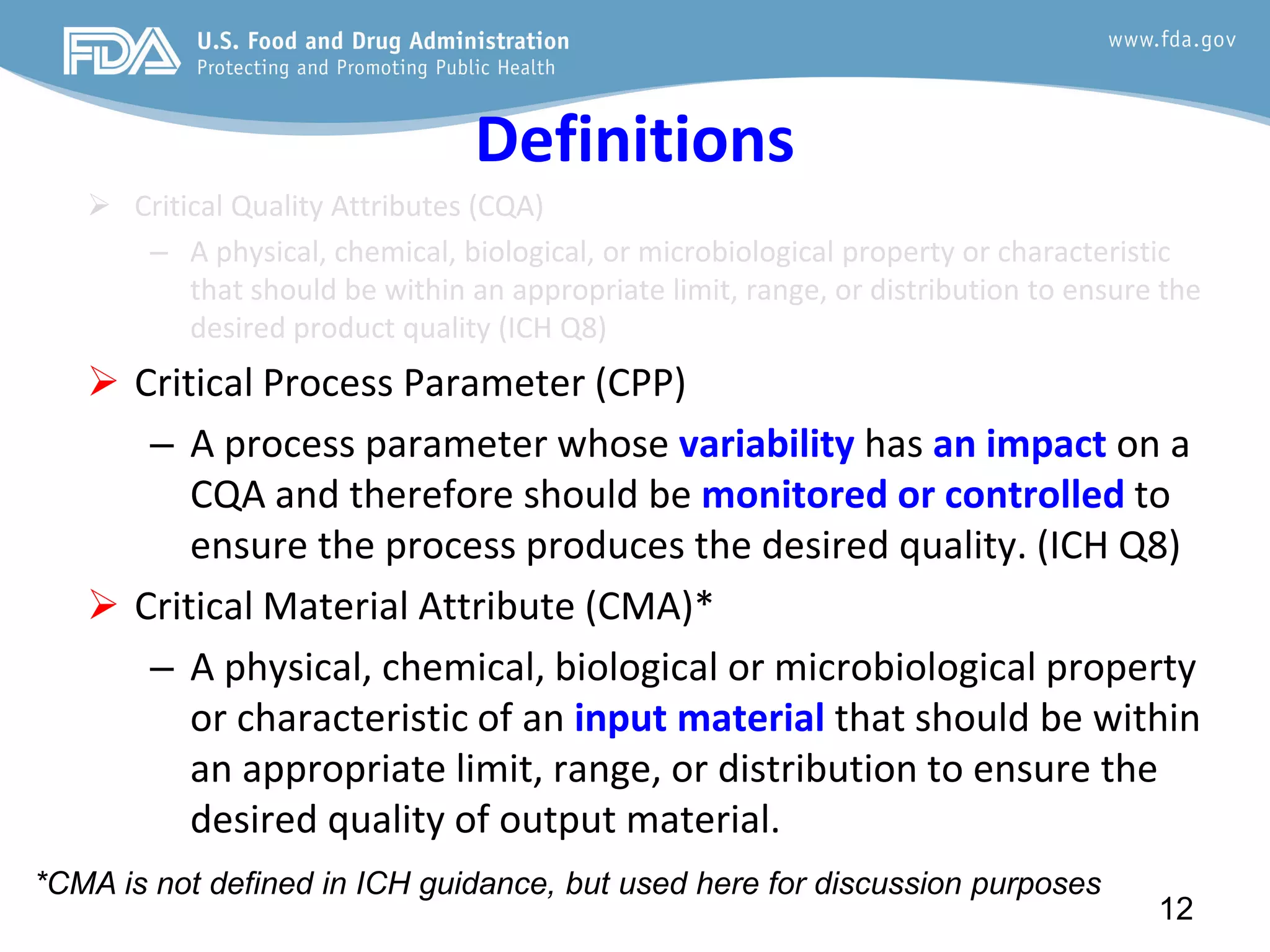
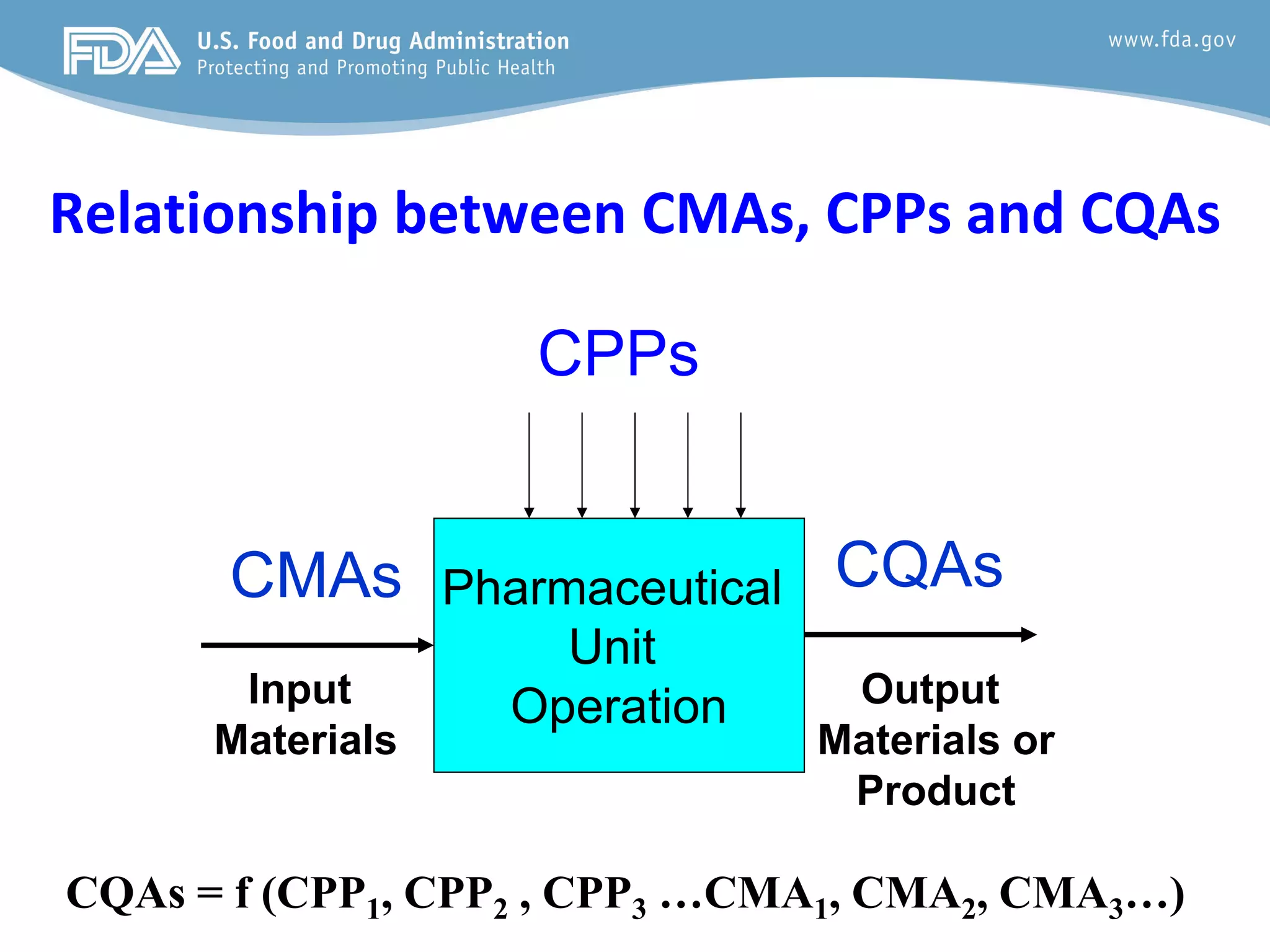
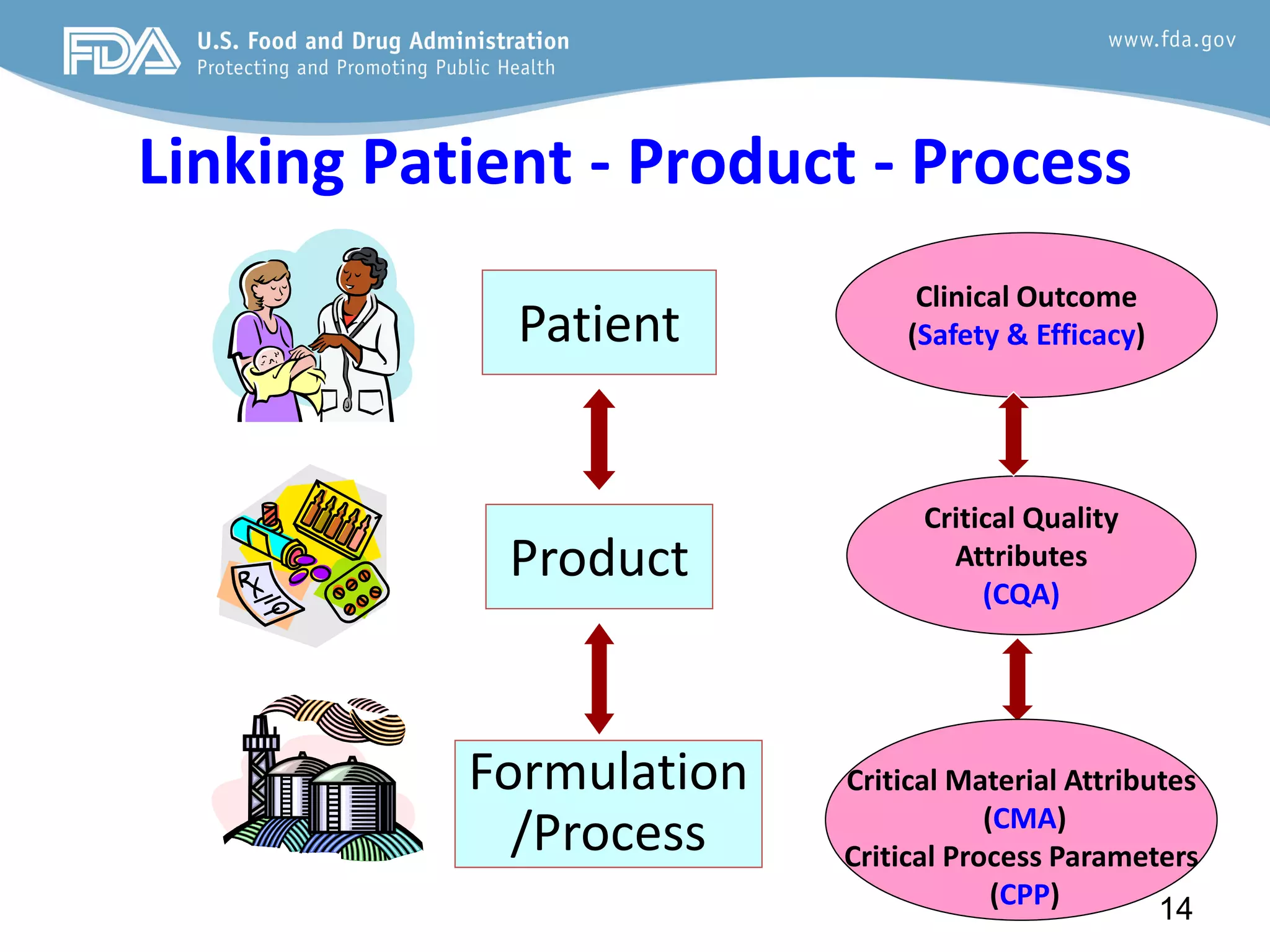
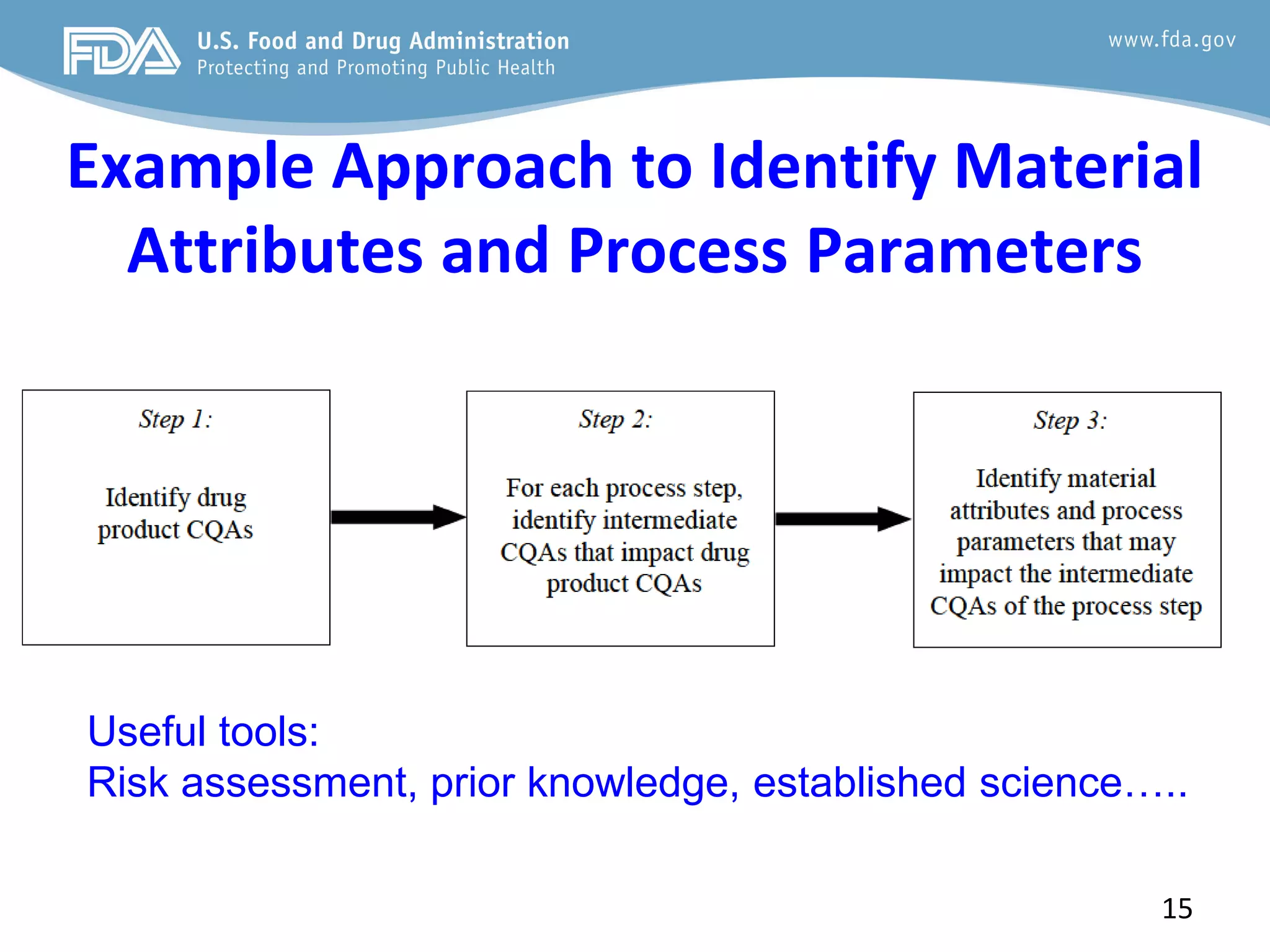
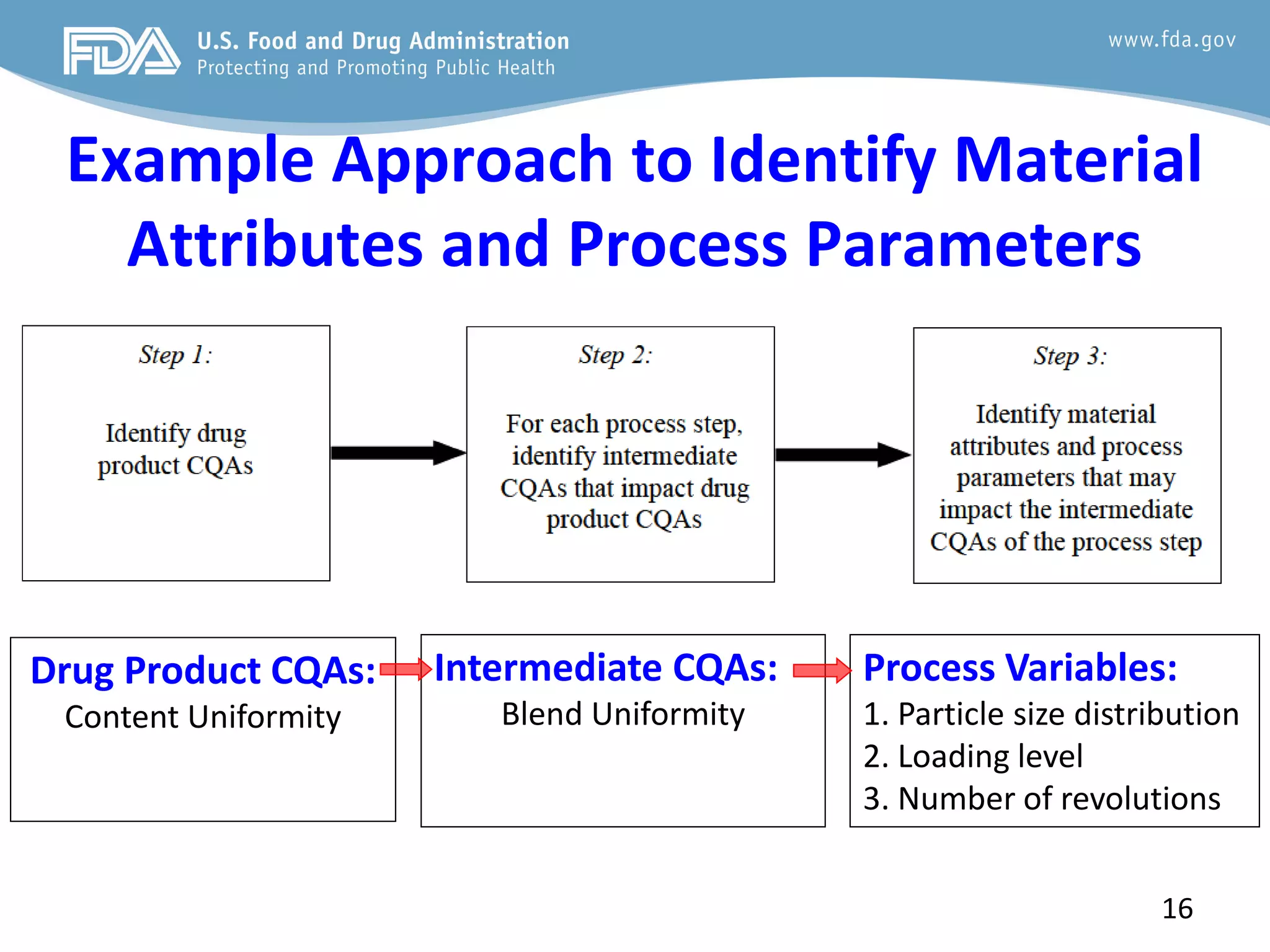
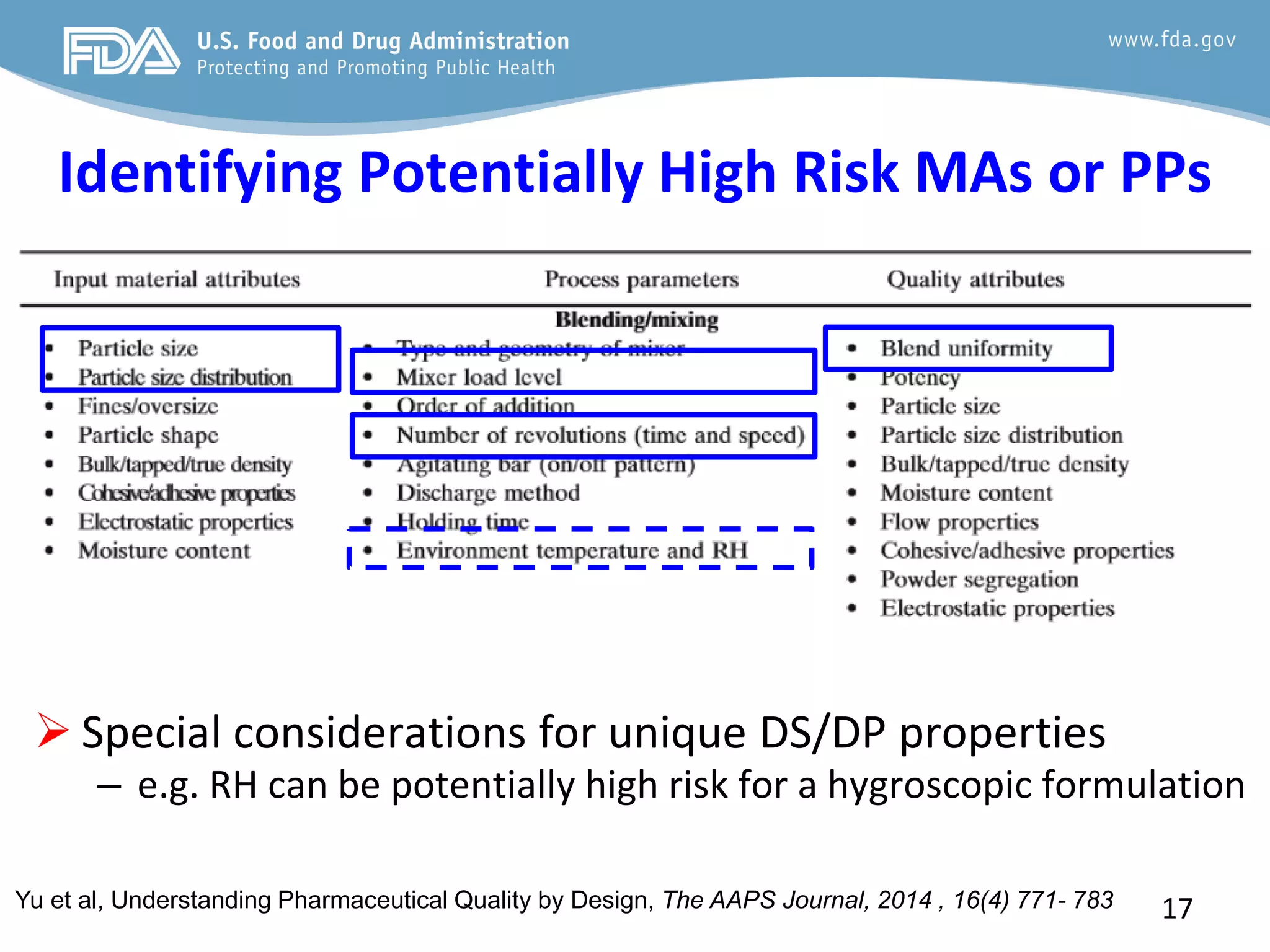
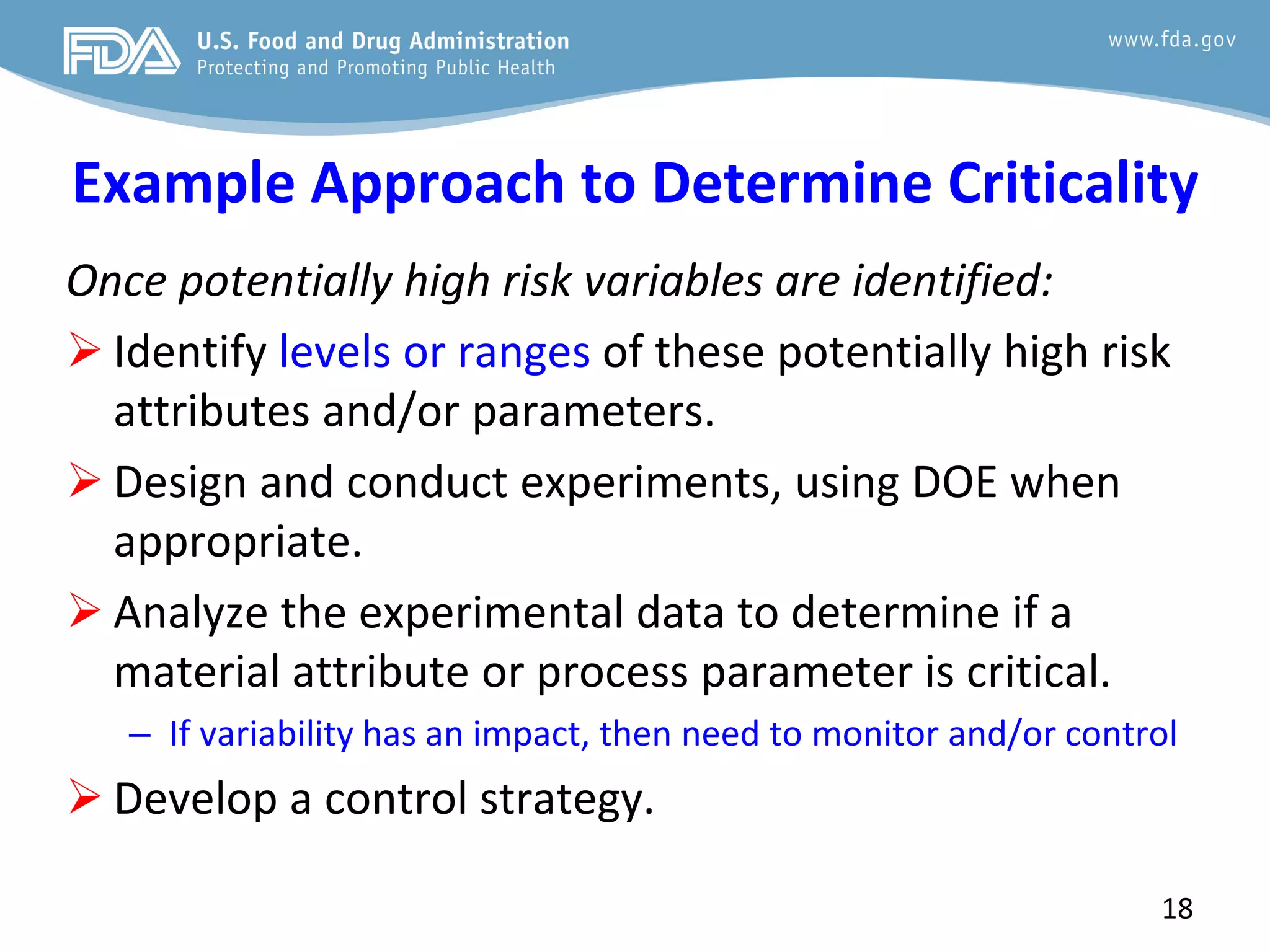
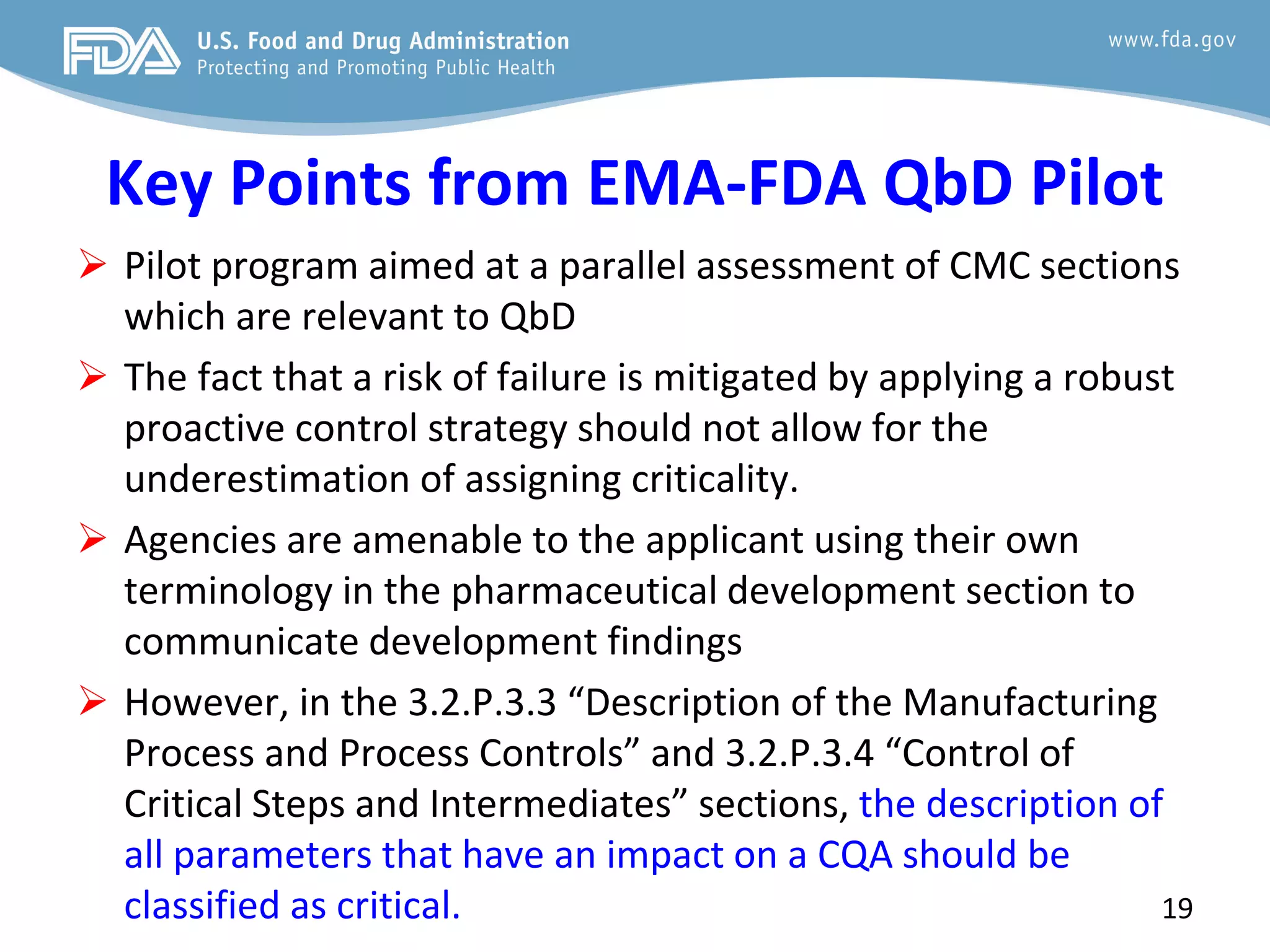
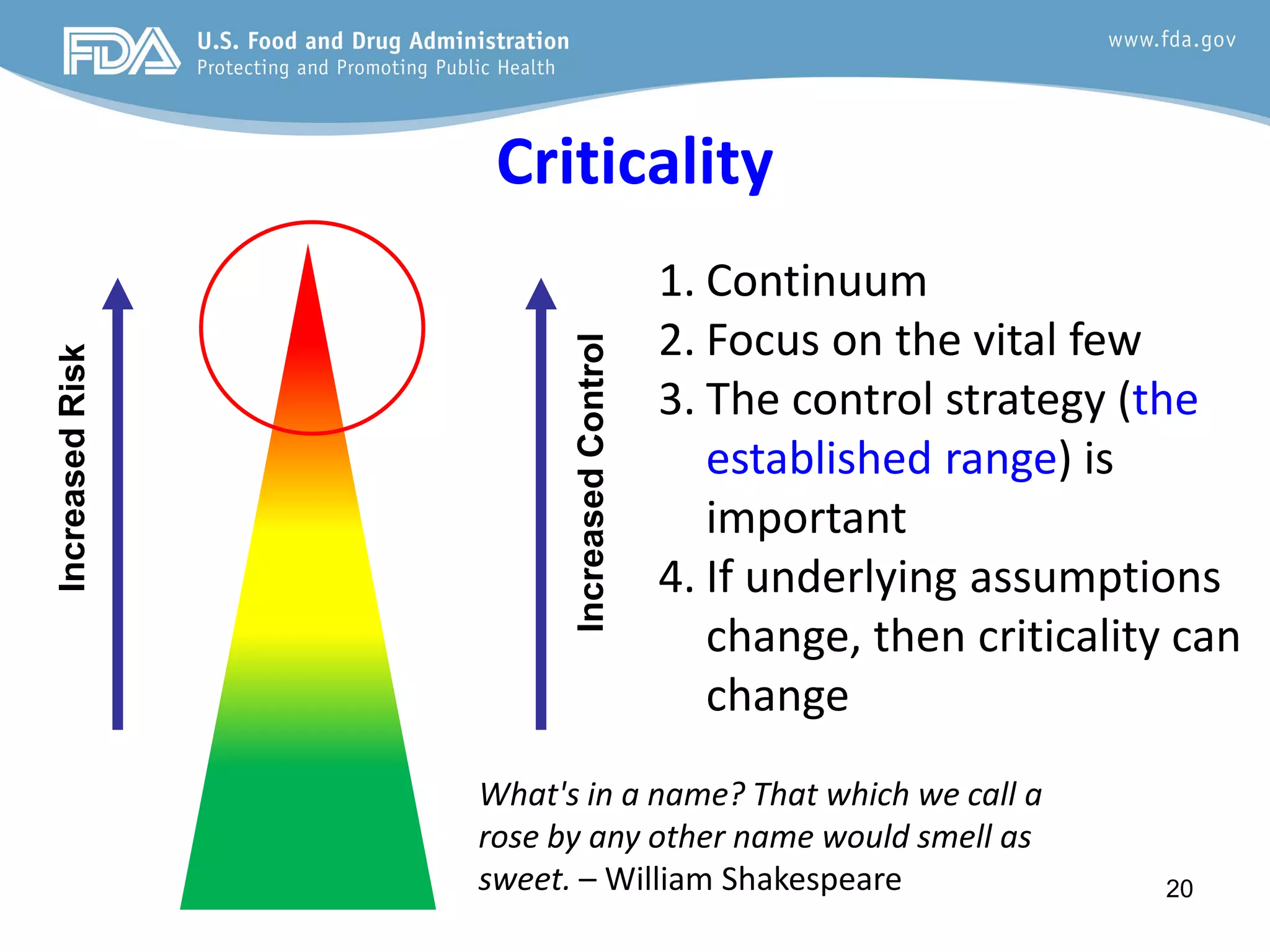
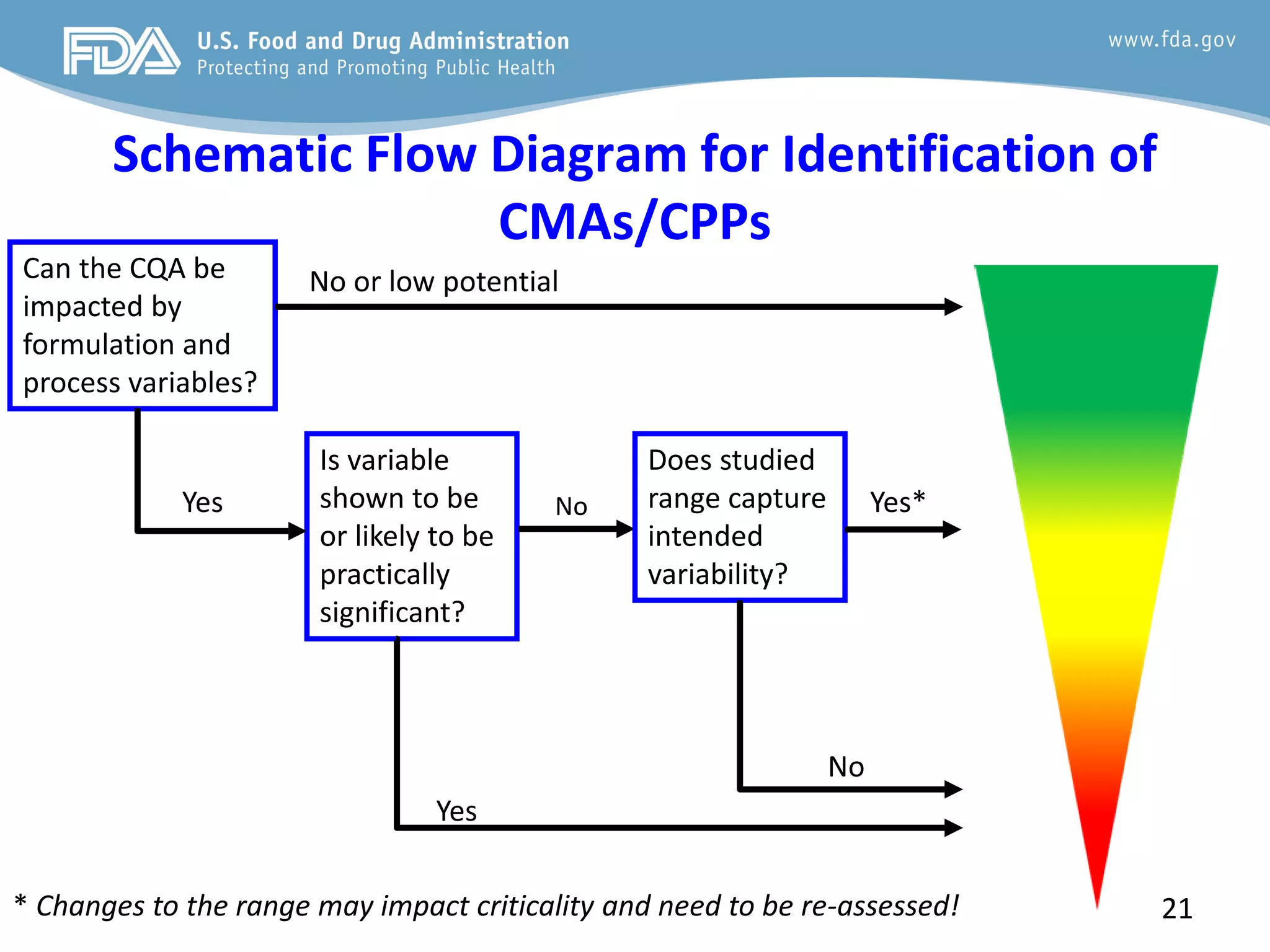
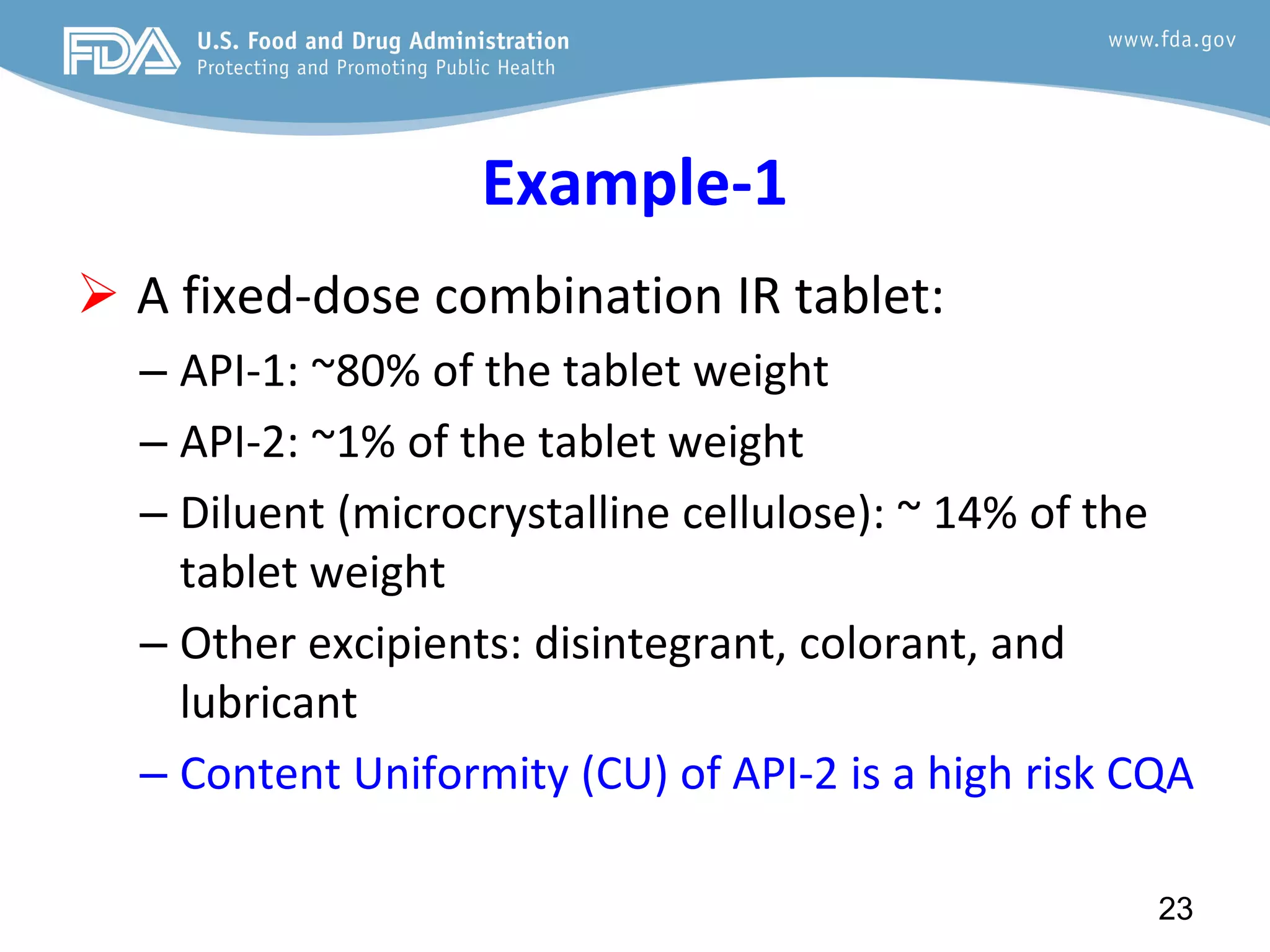
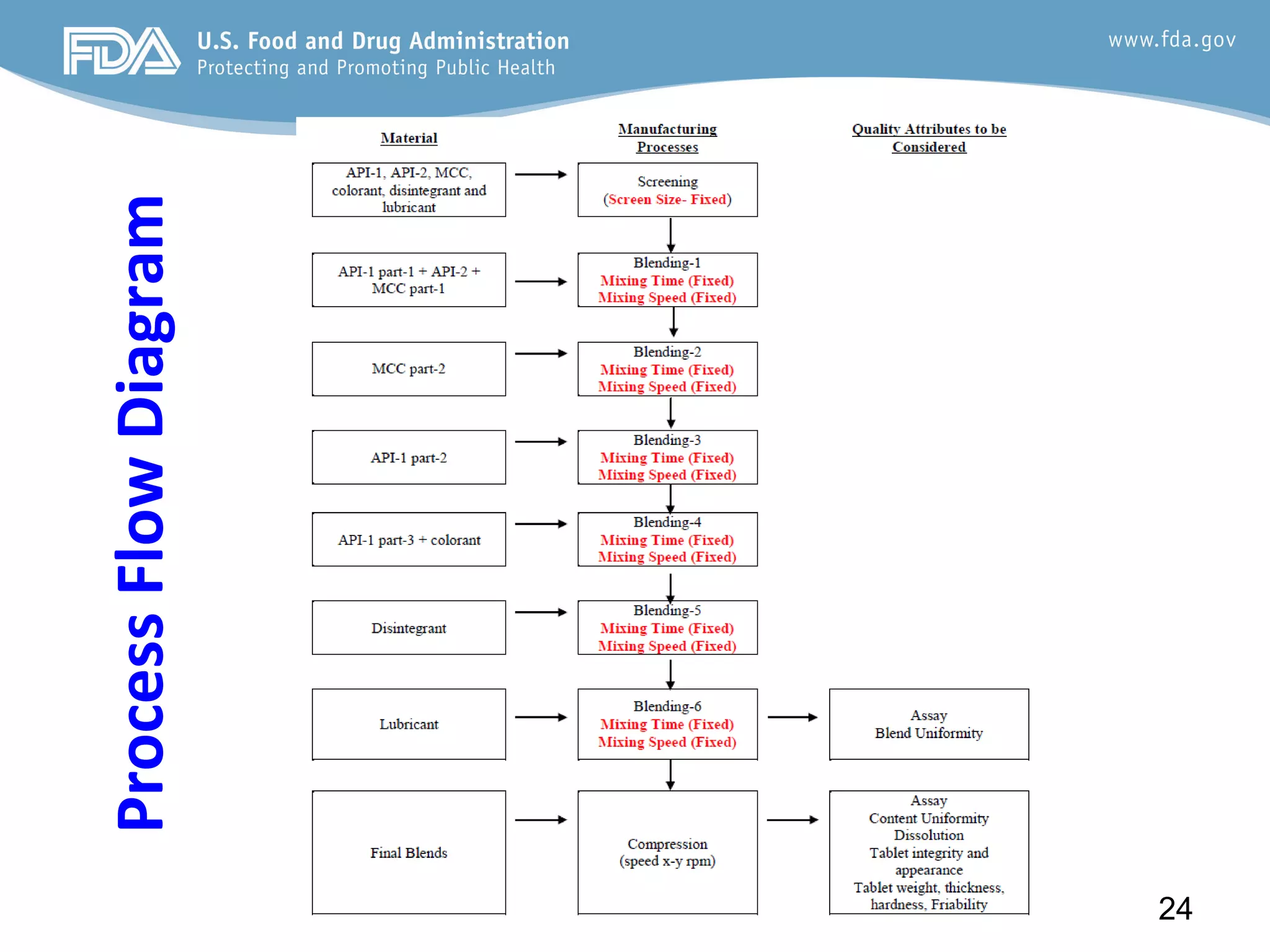
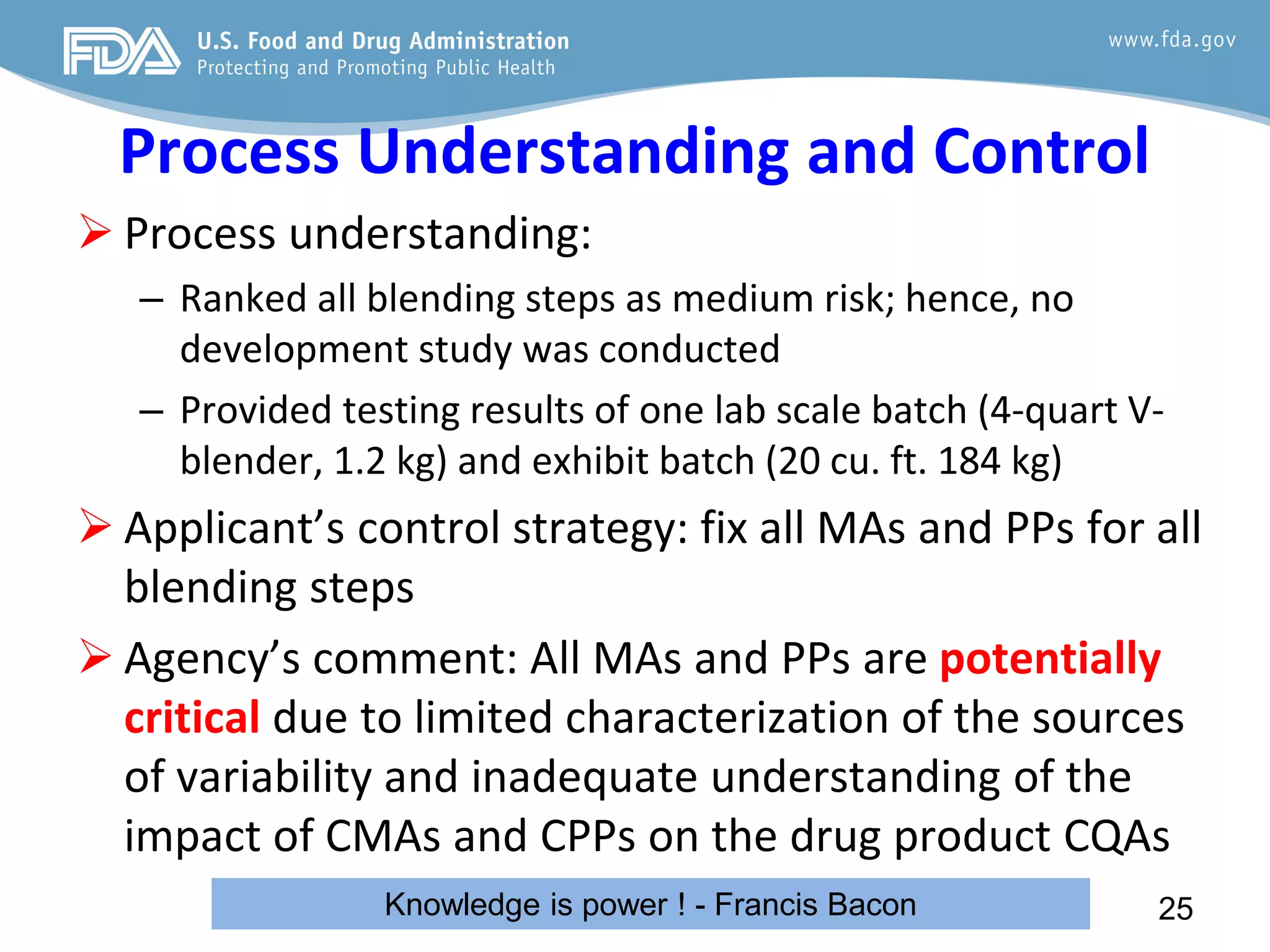
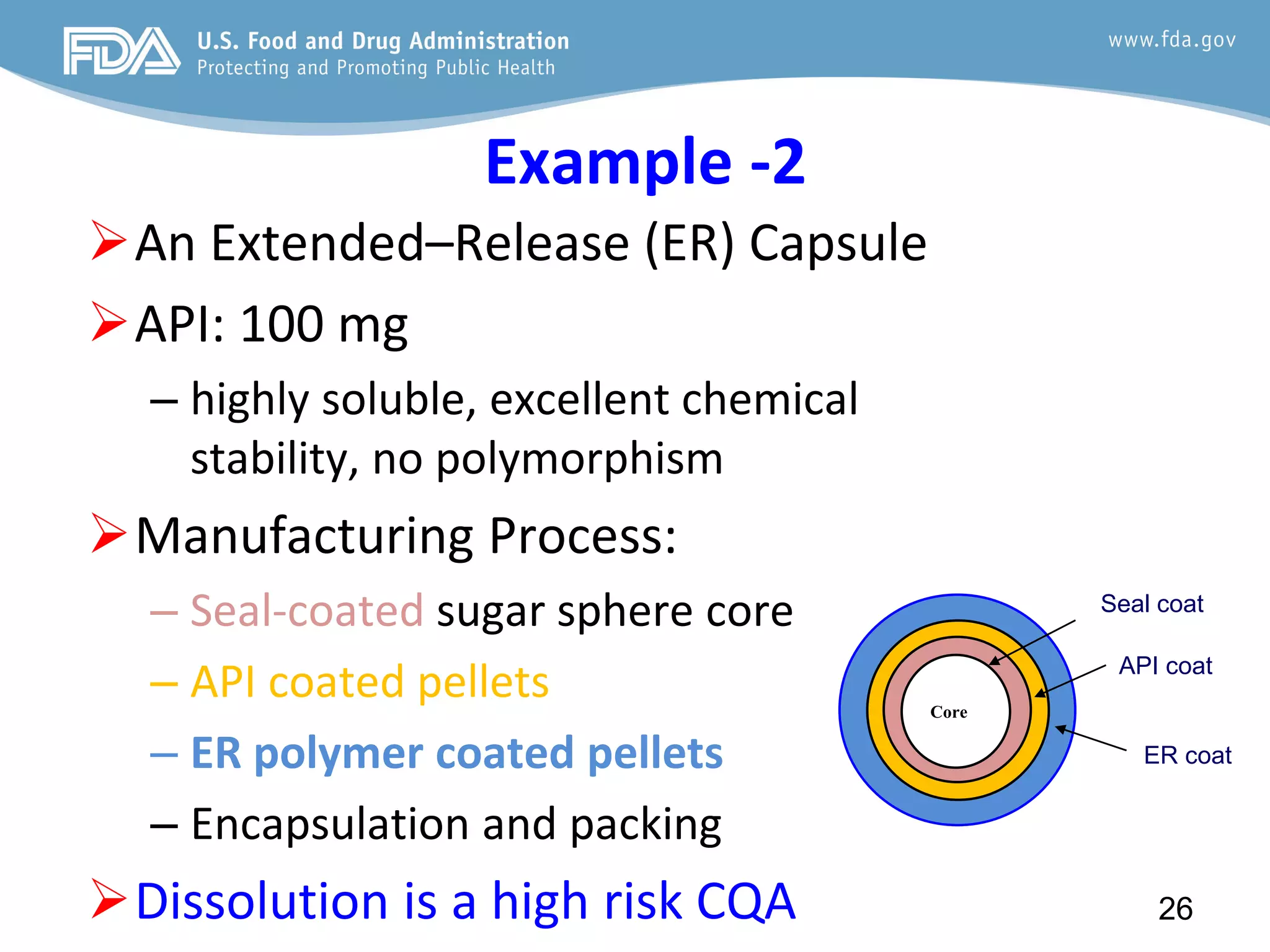
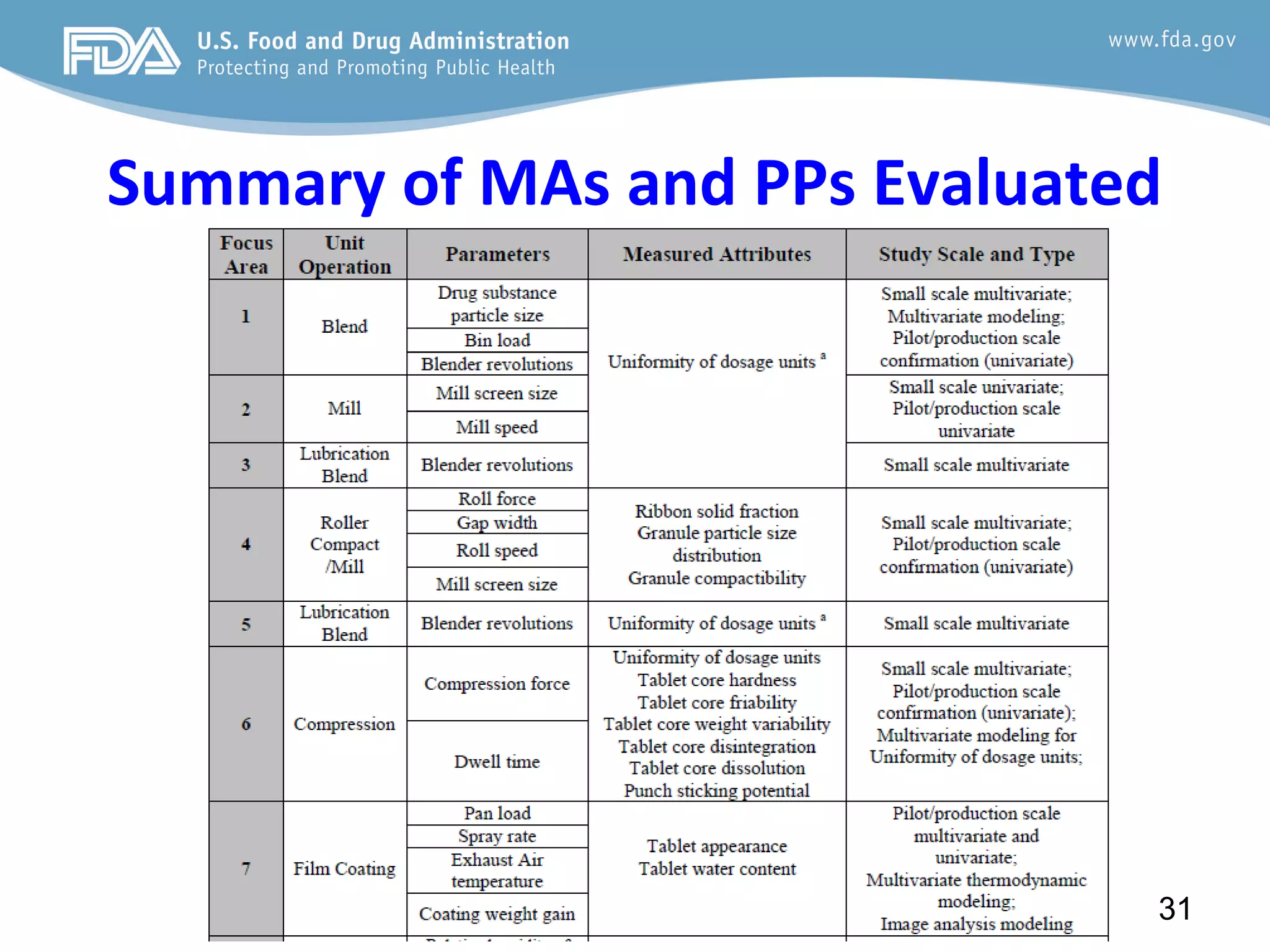
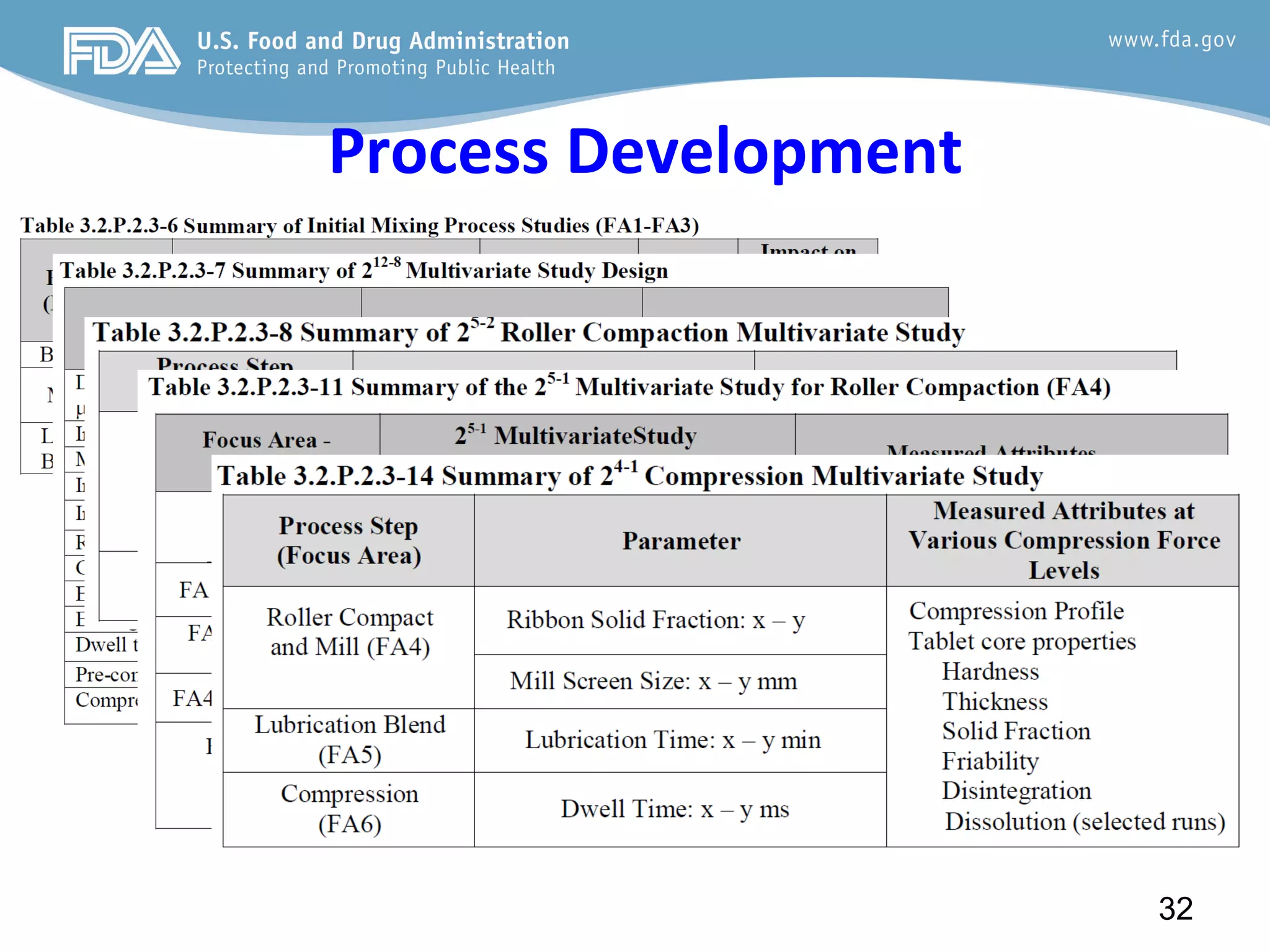
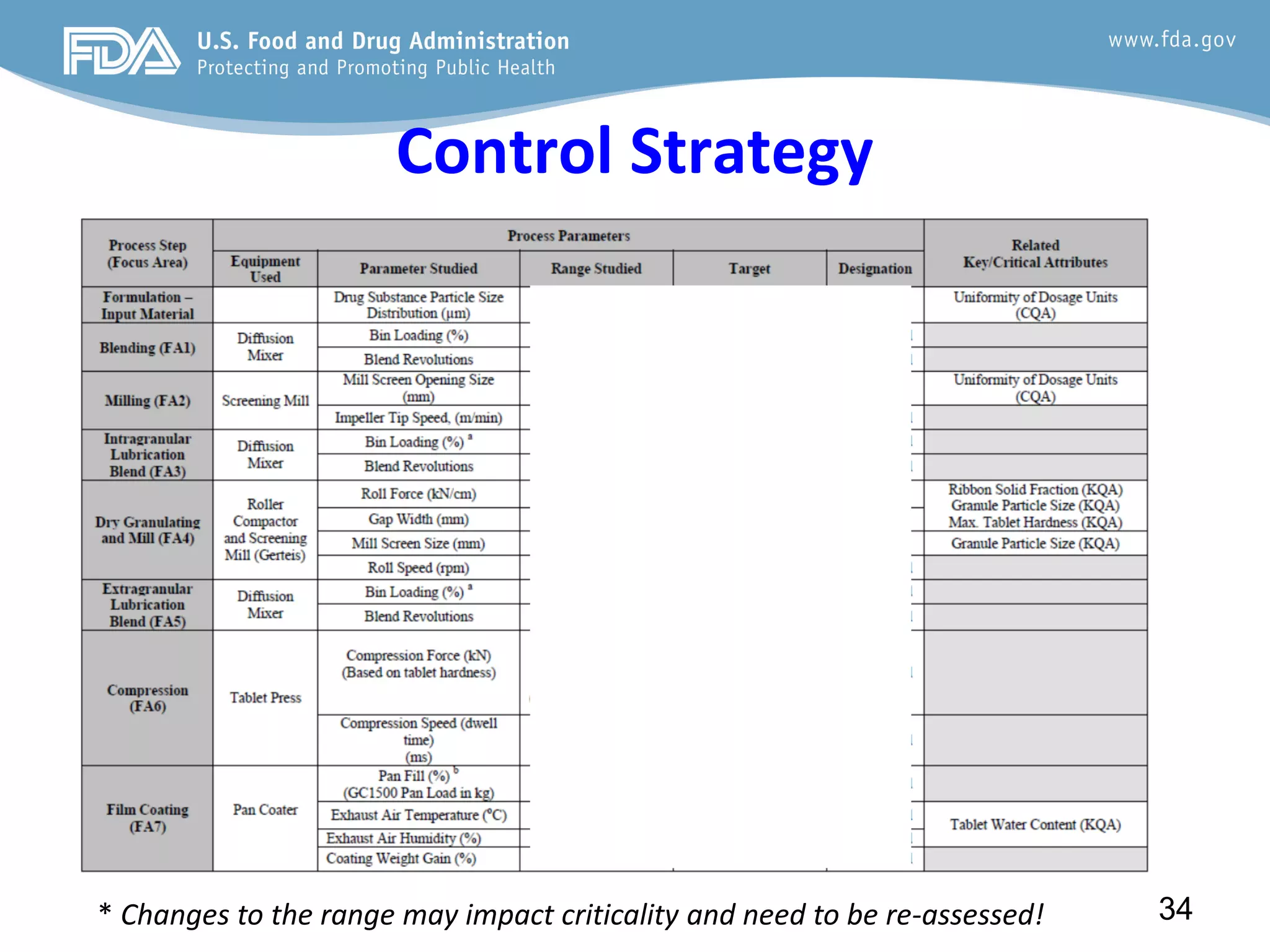
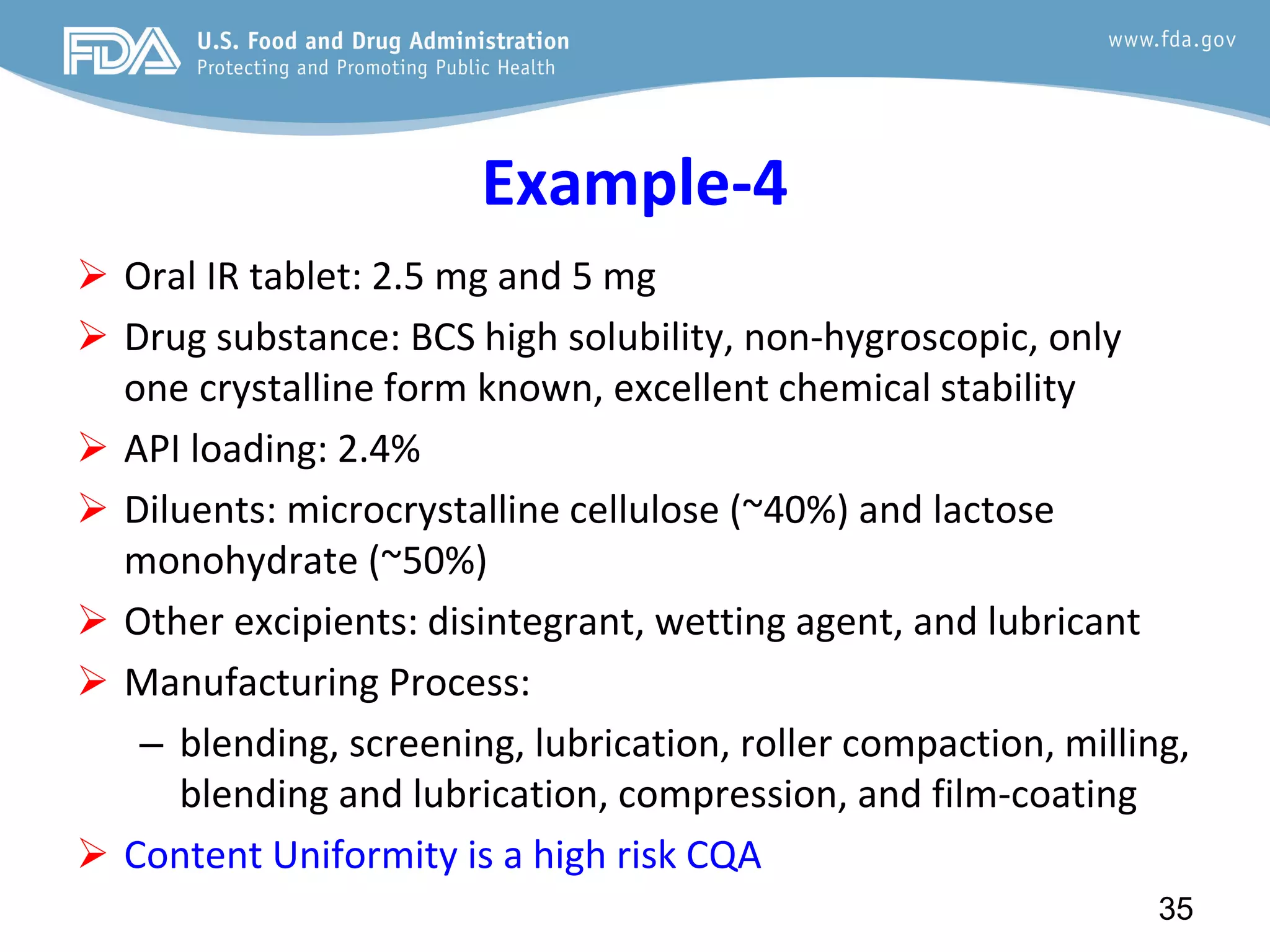
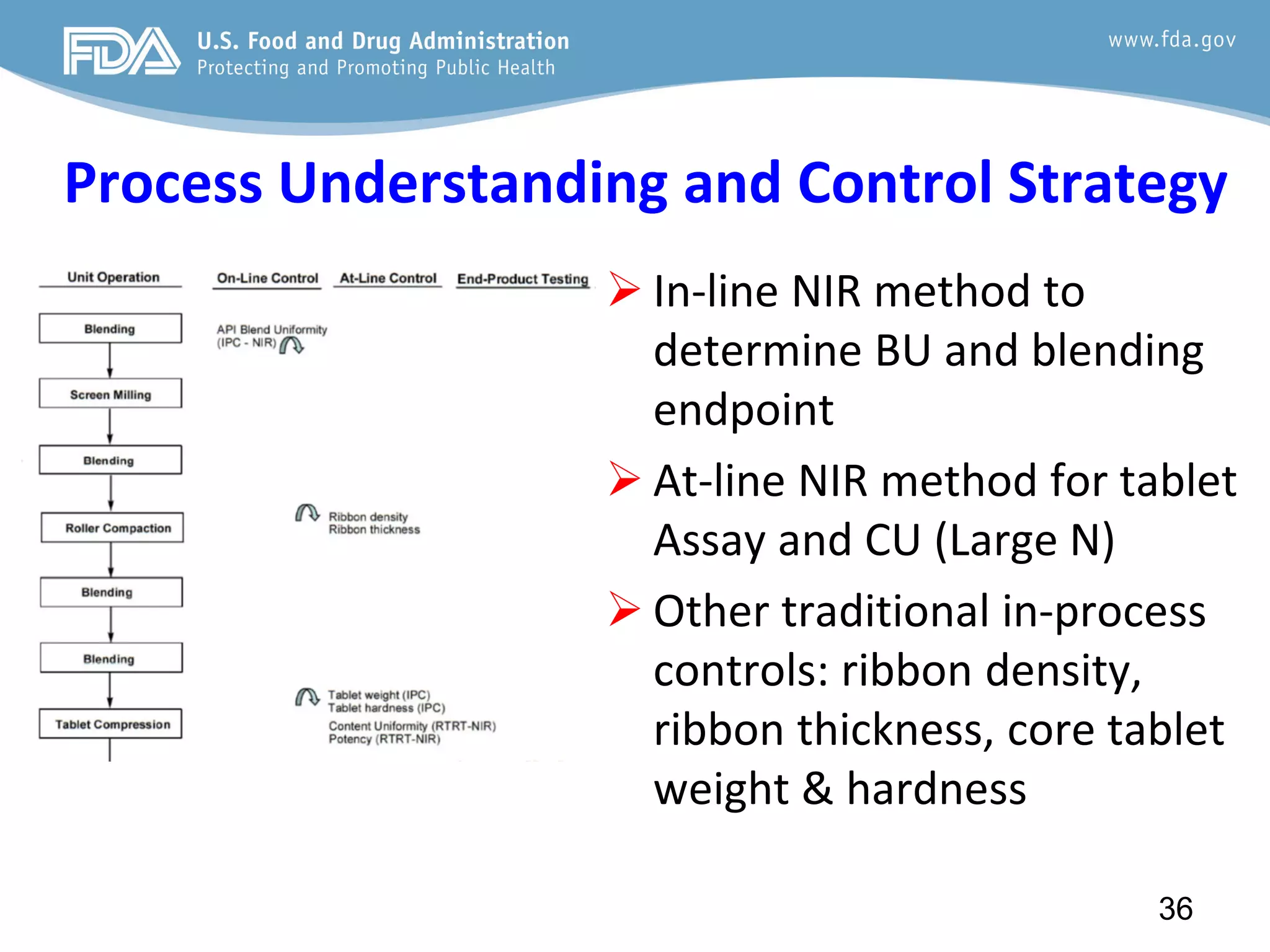
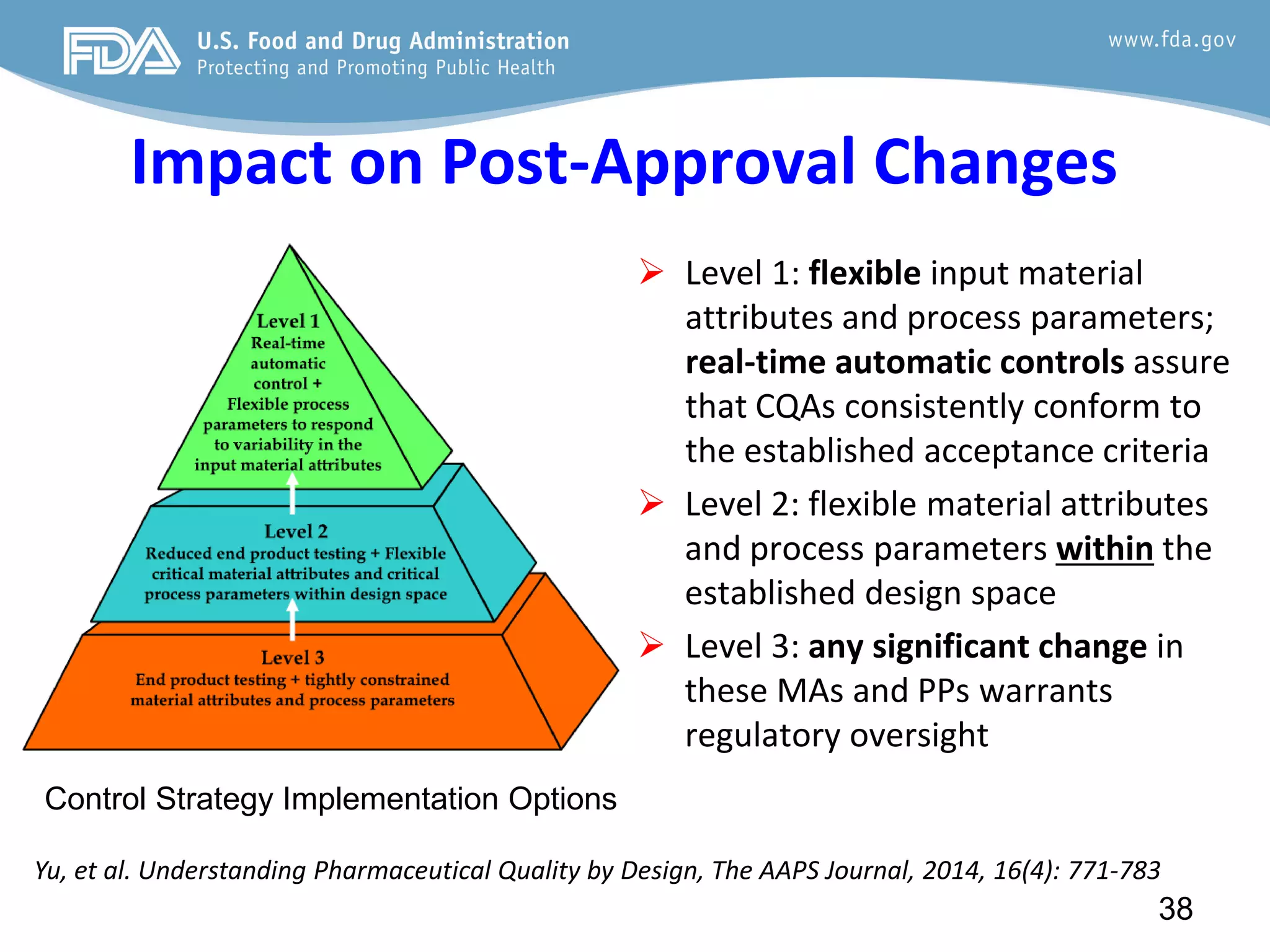
This document provides guidance on identifying critical quality attributes (CQAs), critical material attributes (CMAs), and critical process parameters (CPPs) using a Quality by Design (QbD) approach. It outlines approaches to define the quality target product profile, identify CQAs based on impact to safety and efficacy, and use prior knowledge and risk assessment to identify potentially high risk material and process variables. Experimental design is recommended to determine criticality based on a variable's impact on a CQA. Control strategies should control CMAs and CPPs within studied ranges. The document also provides illustrative examples of applying these approaches to drug products.