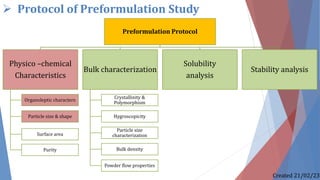
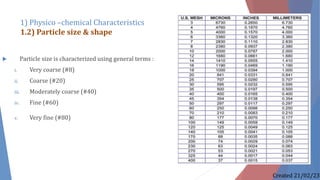
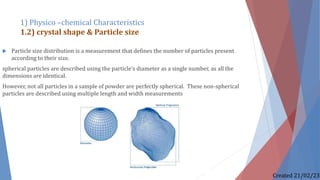
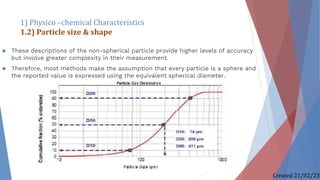
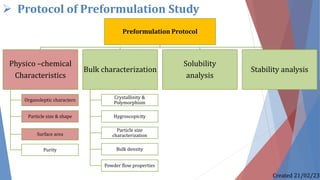
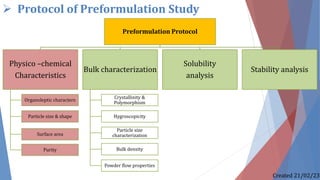
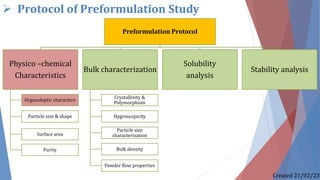
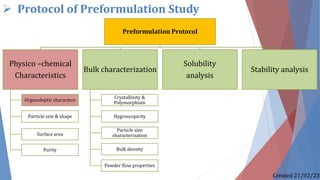
The document discusses preformulation studies, which characterize the physical and chemical properties of a new drug substance to aid in developing a stable, safe, and effective dosage form. The preformulation protocol involves determining physico-chemical characteristics like organoleptic properties, particle size and shape, purity, surface area, bulk characterization, crystallinity, hygroscopicity, and solubility analysis to optimize the drug formulation and dosage form design. Understanding these properties is important for developing an appropriate formulation with excipients and selecting the right manufacturing process.









![ Taste: primary effects of taste are sweet, sour, and salty. There is a close co-relation ship between
chemical structure and taste.
Sour taste is due to acidic nature.
▪ Sour taste [H+] and lipid solubility of substance i.e., acids get ionized in aqueous solution(it depends on H+ ion
concentration) examples: Lemon, Vinegar, Citric acid, Malic acid, Apple.
Salty taste is due to cationic species, halide salts.
▪ Example: Sodium chloride, sodium bromide, sodium iodide. Increase in molecular weight of halide results in
increase in bitter taste. example: potassium bromide, Ammonium salts.
▪ Sodium chloride is salty, whereas , potassium chloride is bitter.
Sweet taste is due to polyhydroxy compounds.
▪ Sweet taste [OH-] and aqueous solubility. Example: sugar, glycerin, alpha amino acids, etc.
1) Physico –chemical Characteristics
1.1) Organoleptic characters
Created 21/02/23](https://image.slidesharecdn.com/preformulationstudiespartibyemanateia00-230902142938-7ffaf69d/85/Preformulation-studies-Part-I-by-Eman-Ateia_00-pdf-12-320.jpg)