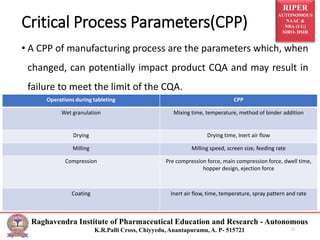
The document presents information on Quality by Design (QbD), a systematic approach to pharmaceutical development that emphasizes product and process understanding. It defines key QbD concepts like target product profile, quality target product profile, critical quality attributes, critical material attributes, and critical process parameters. The benefits of QbD for industry include eliminating batch failures and empowering technical staff. Design space, design of experiments, and process analytical techniques are important tools in QbD. Regulatory agencies support the QbD approach for developing scientific understanding and continuous improvement.