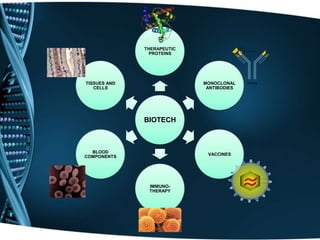
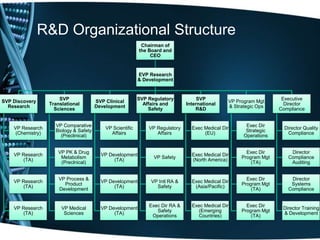
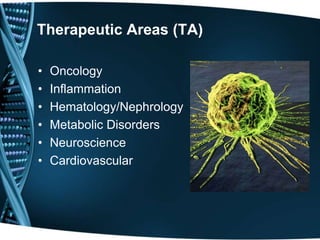
The document provides an overview of drug development and the biotechnology industry. It discusses the traditional and contemporary business models in the industry, which have shifted from closed innovation to more open innovation. It also outlines the services offered by one company, including clinical development support and regulatory consultancy. The document describes the company's research interests in areas like vaccines, proteins, and monoclonal antibodies. It includes organizational charts and provides details about the company's project pipeline across various phases of drug development. Finally, it discusses challenges in the industry like high costs and manufacturing techniques for cost reduction.