The document discusses preformulation studies, which characterize the physicochemical properties of drug substances. Some key points:
- Preformulation studies are conducted early in drug development to understand how a drug's properties will influence formulation, stability, bioavailability and the development process.
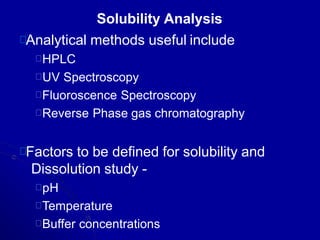
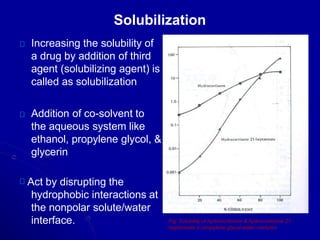
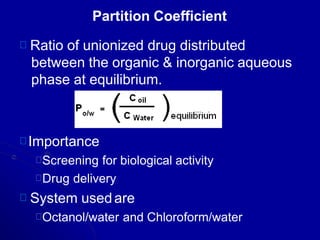
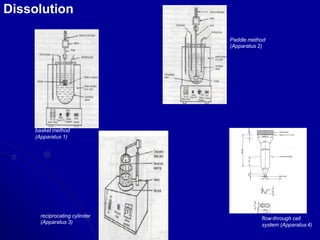
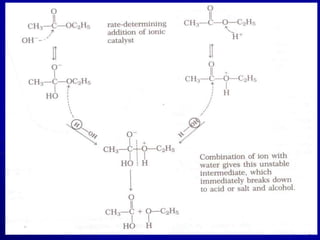
- Areas of study include bulk properties, solubility, stability and more. Properties like solubility, dissolution, polymorphism can impact the drug's performance.
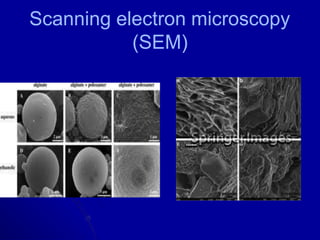
- Various analytical techniques are used to characterize aspects like crystallinity, particle size, hygroscopicity and more which provide essential information for designing drug products.
- Understanding a drug's properties is important for selecting excipients, manufacturing processes, container closure systems and developing analytical