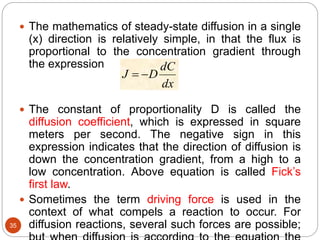
Diffusion bonding is a solid-state welding technique that joins materials together through atomic diffusion without melting. It involves applying high pressure and moderate heat to join carefully cleaned and mated surfaces. Diffusion occurs in two stages - initial metal-to-metal contact formation followed by atomic diffusion and grain growth across the interface to form a complete bond. Various factors like temperature, pressure, time and surface preparation influence the diffusion rate. Common diffusion bonding methods include gas pressure bonding, vacuum fusion bonding and eutectic bonding. Diffusion bonding finds applications in the fabrication of components for industries like aerospace, nuclear and others.