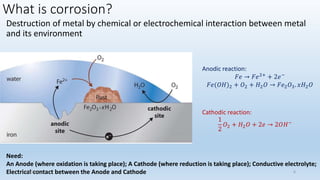
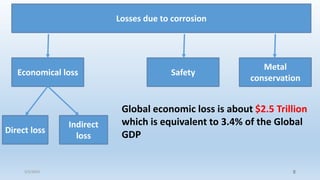
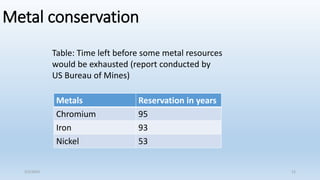
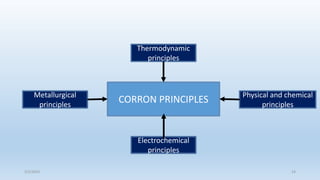
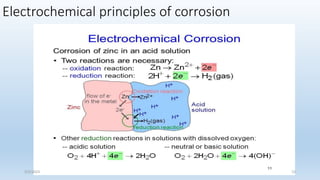
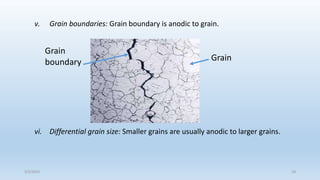
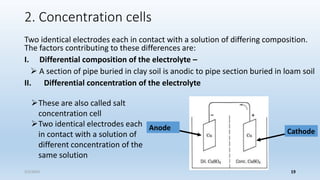
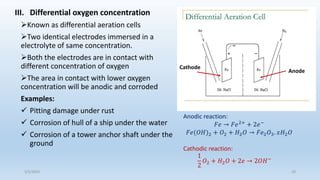
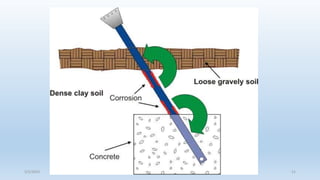
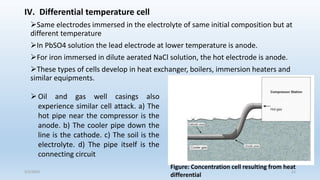
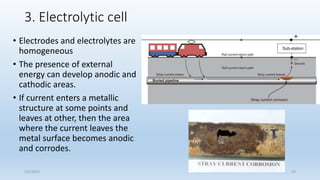
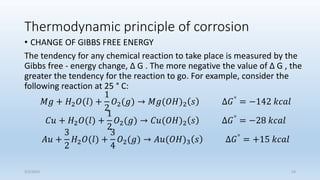
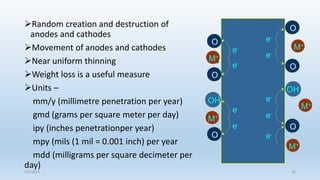
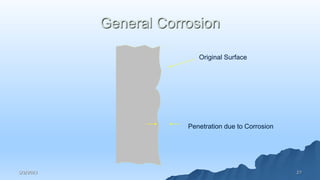
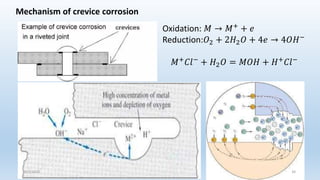
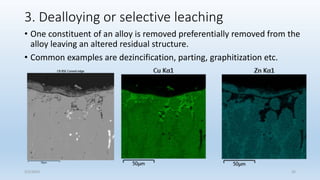
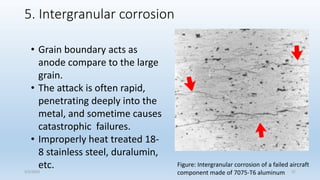
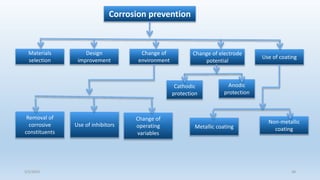
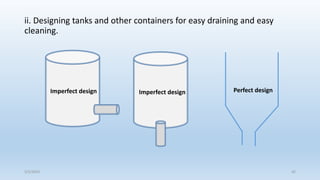
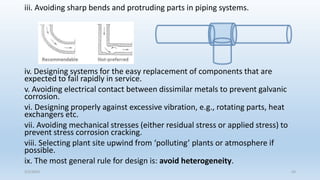
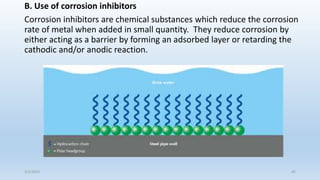
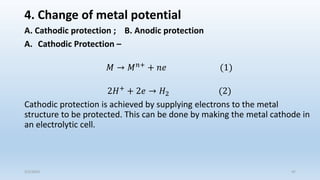
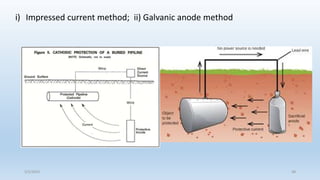
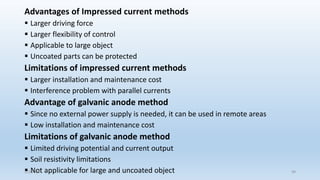
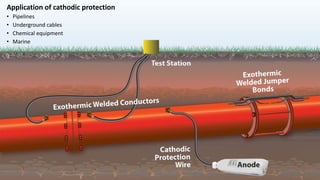
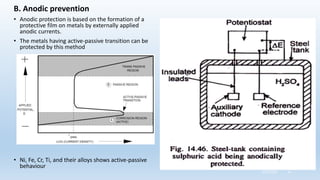
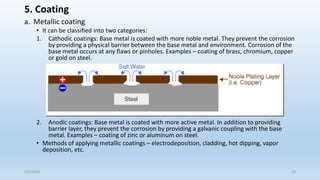
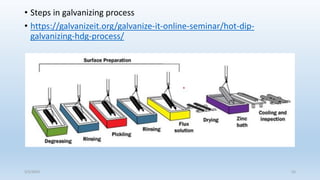
This document discusses corrosion, its causes, impacts, and prevention methods, emphasizing economic losses due to corrosion estimated at $2.5 trillion globally. It outlines different types of corrosion, the processes involved, and the role of corrosion engineers in mitigating these issues. Additionally, it covers material selection, design improvements, and various chemical inhibitors as strategies for corrosion prevention.