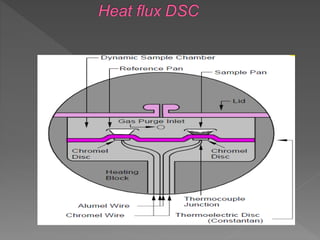
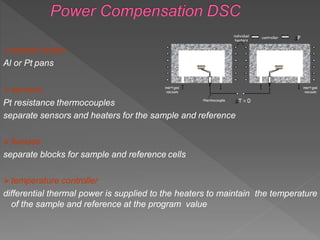
This document discusses differential scanning calorimetry (DSC), a thermal analysis technique where the temperature and heat flow of a sample are measured as it is subjected to a controlled temperature program. DSC provides quantitative and qualitative data on physical and chemical changes that involve endothermic or exothermic processes, such as phase transitions, melting points, heat capacity, and oxidation. The document outlines the components of a DSC instrument and how it works, as well as applications of DSC in various fields including polymers, pharmaceuticals, and biochemistry.