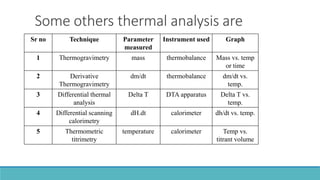
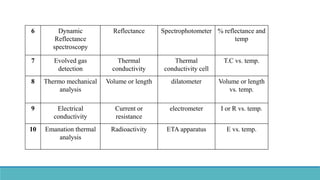
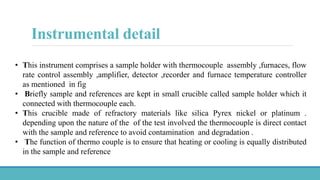
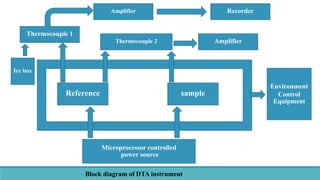
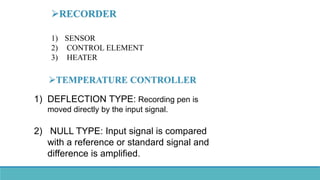
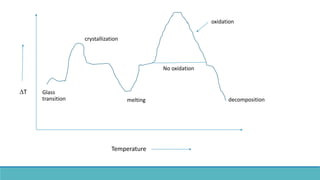
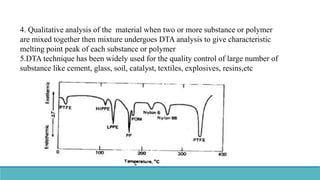
Thermal analysis techniques such as differential thermal analysis (DTA) measure the temperature difference between a sample and an inert reference material as they are heated or cooled. DTA can identify physical and chemical changes in materials through endothermic or exothermic peaks on a thermogram. The DTA instrument contains sample and reference holders connected to thermocouples which are heated in a furnace and temperature differences are amplified, detected and recorded. DTA has applications in determining melting points, phase changes, and reactions, as well as characterizing materials like polymers, pharmaceuticals, soils and catalysts. It can also provide information on purity, thermal stability and kinetic parameters of organic materials.