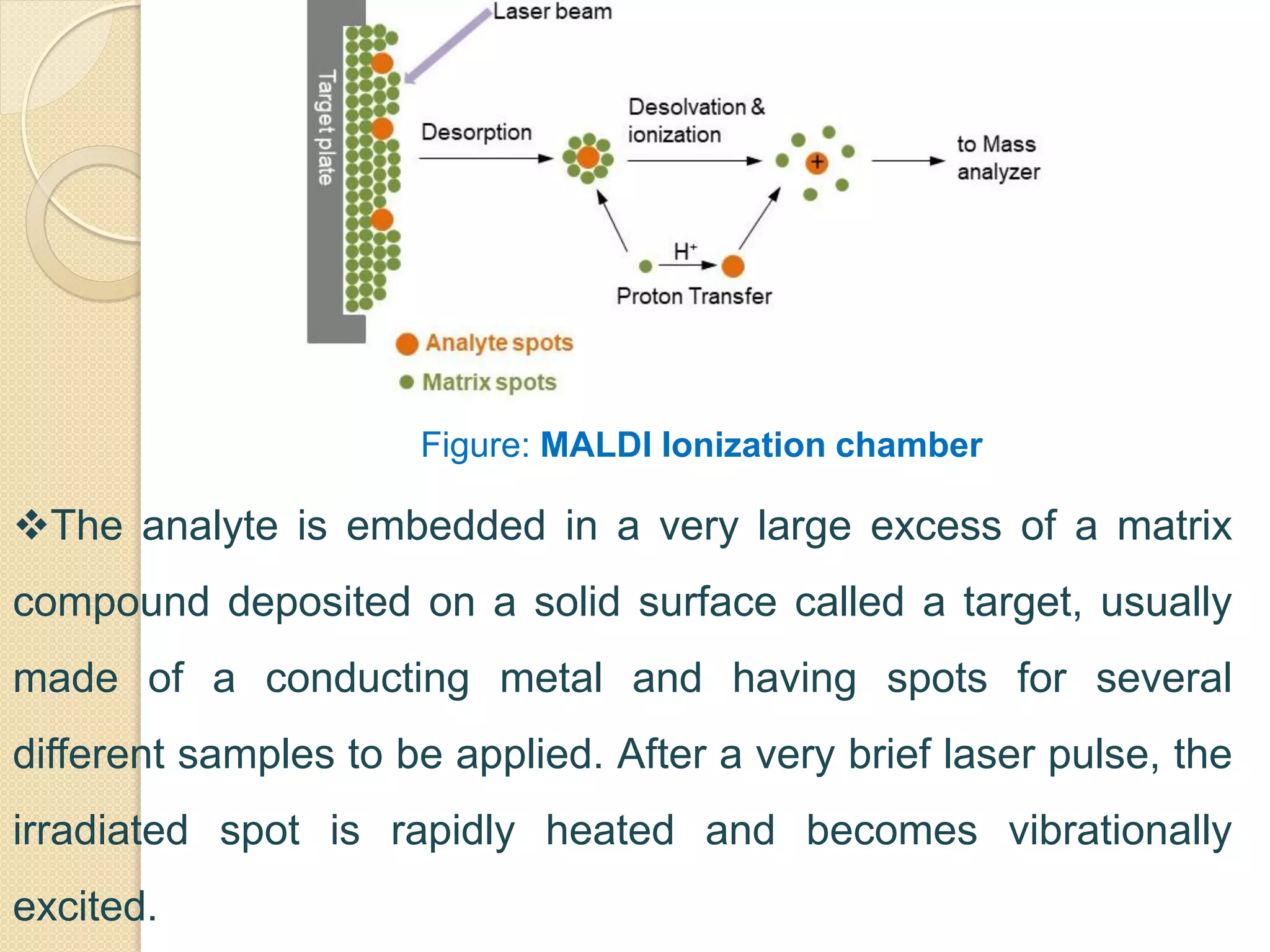
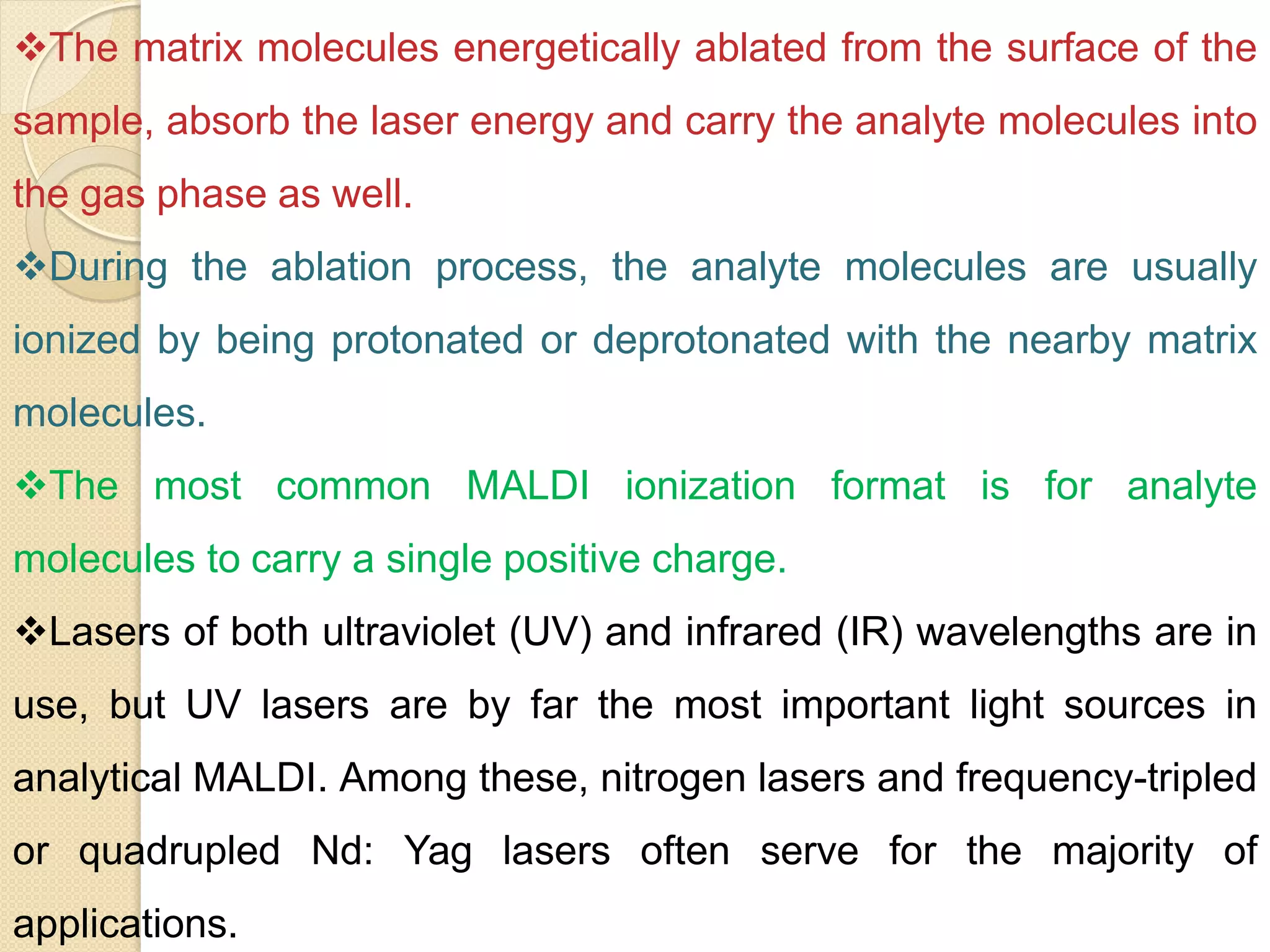
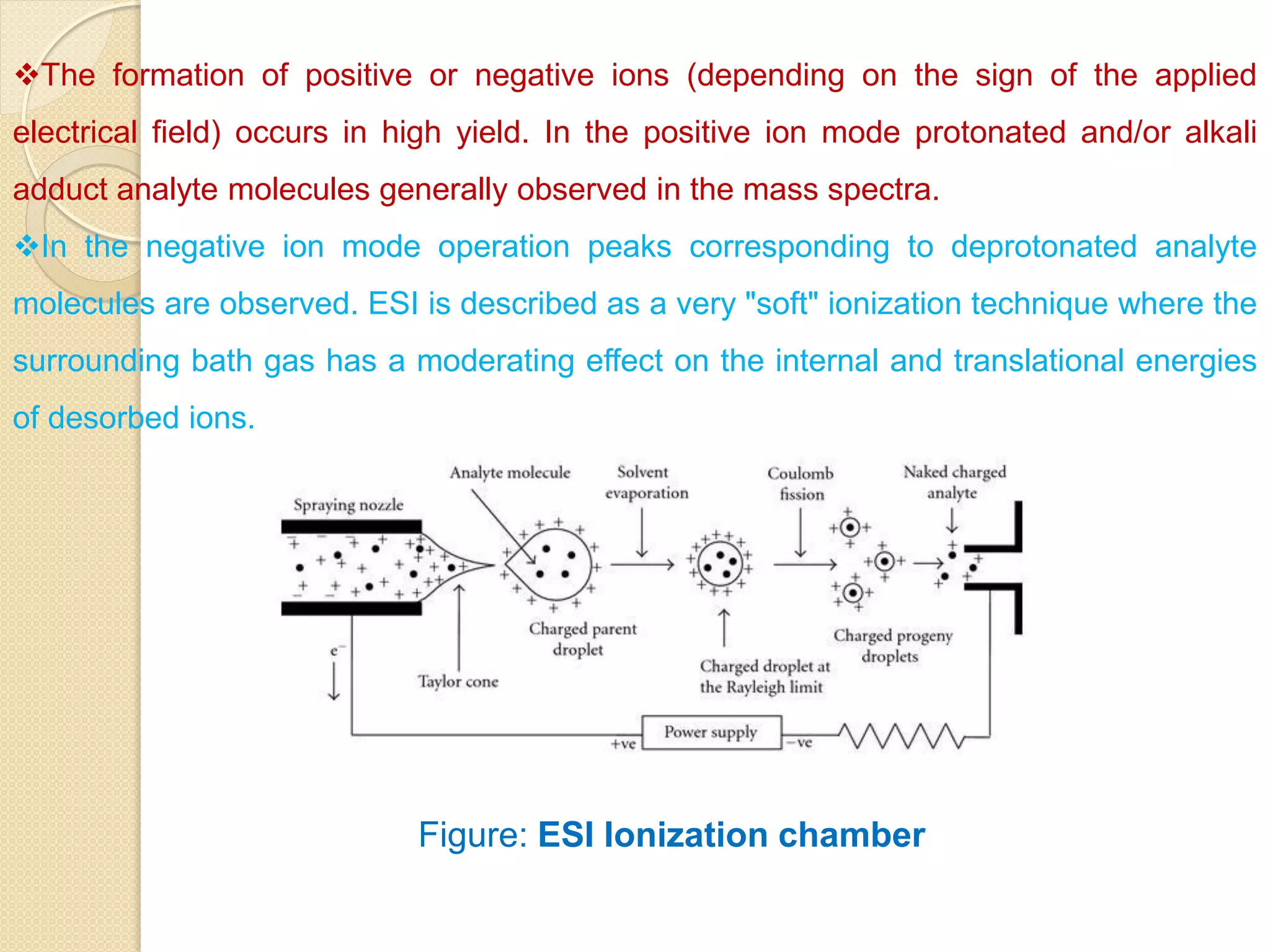
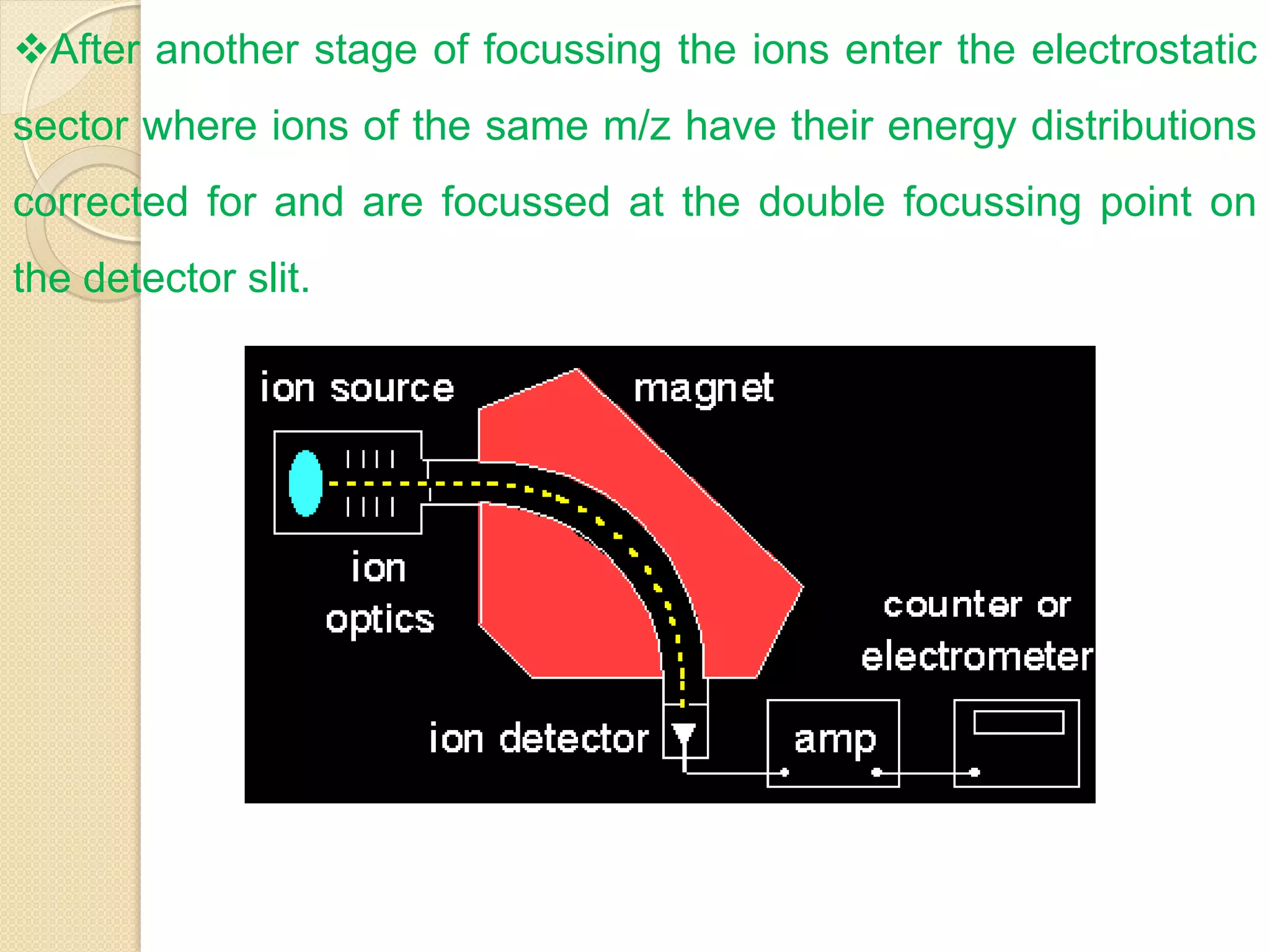
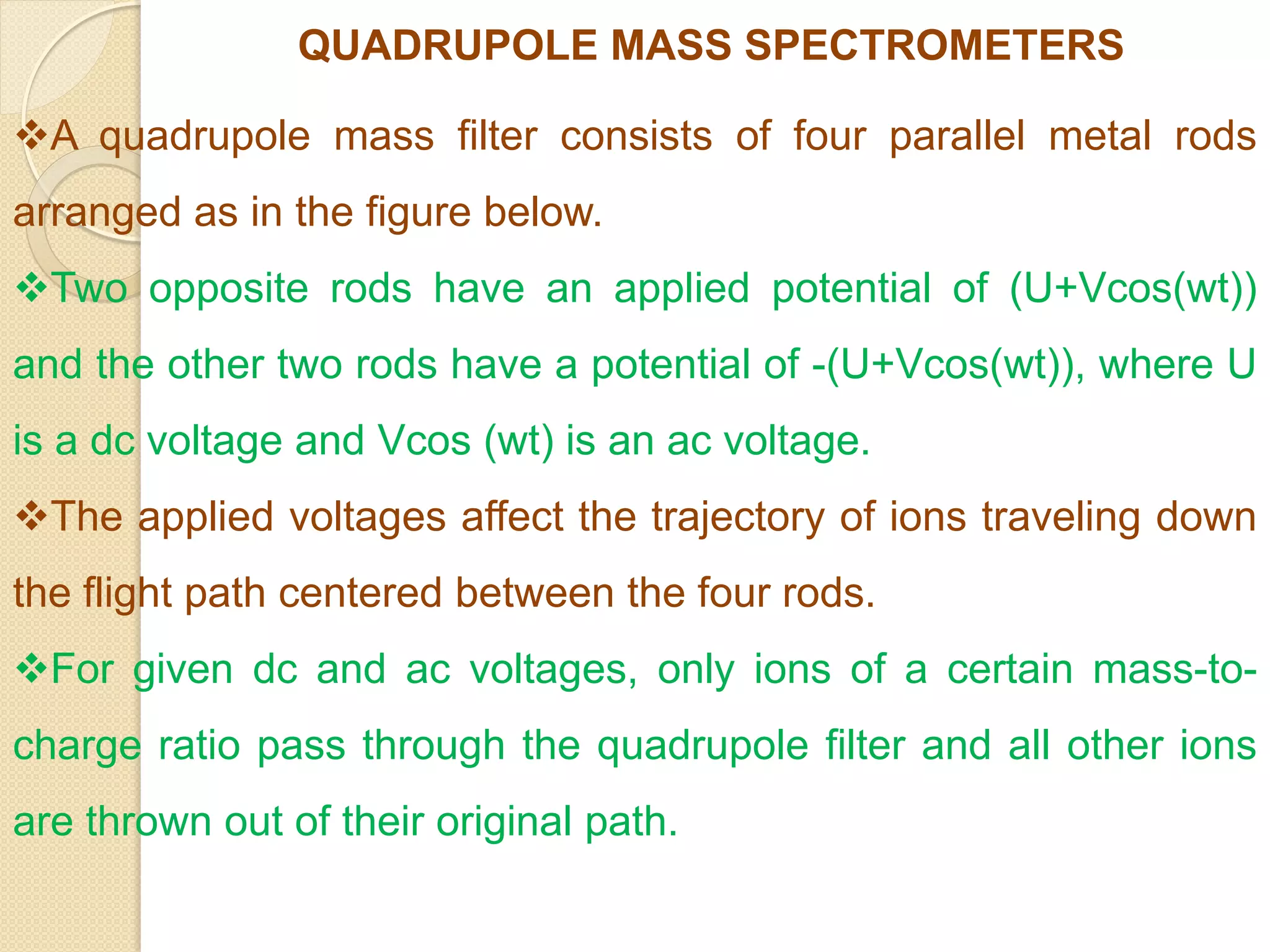
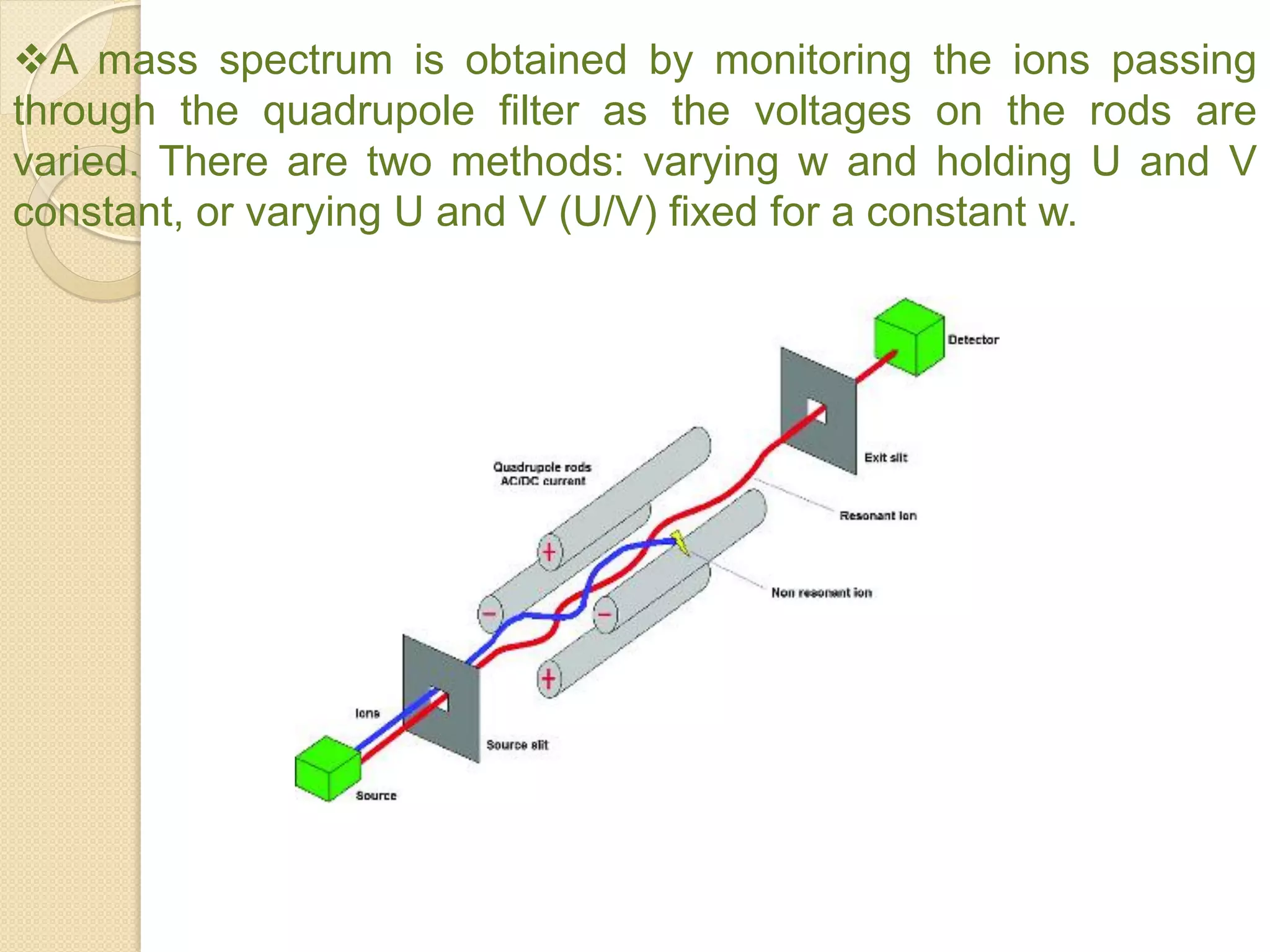
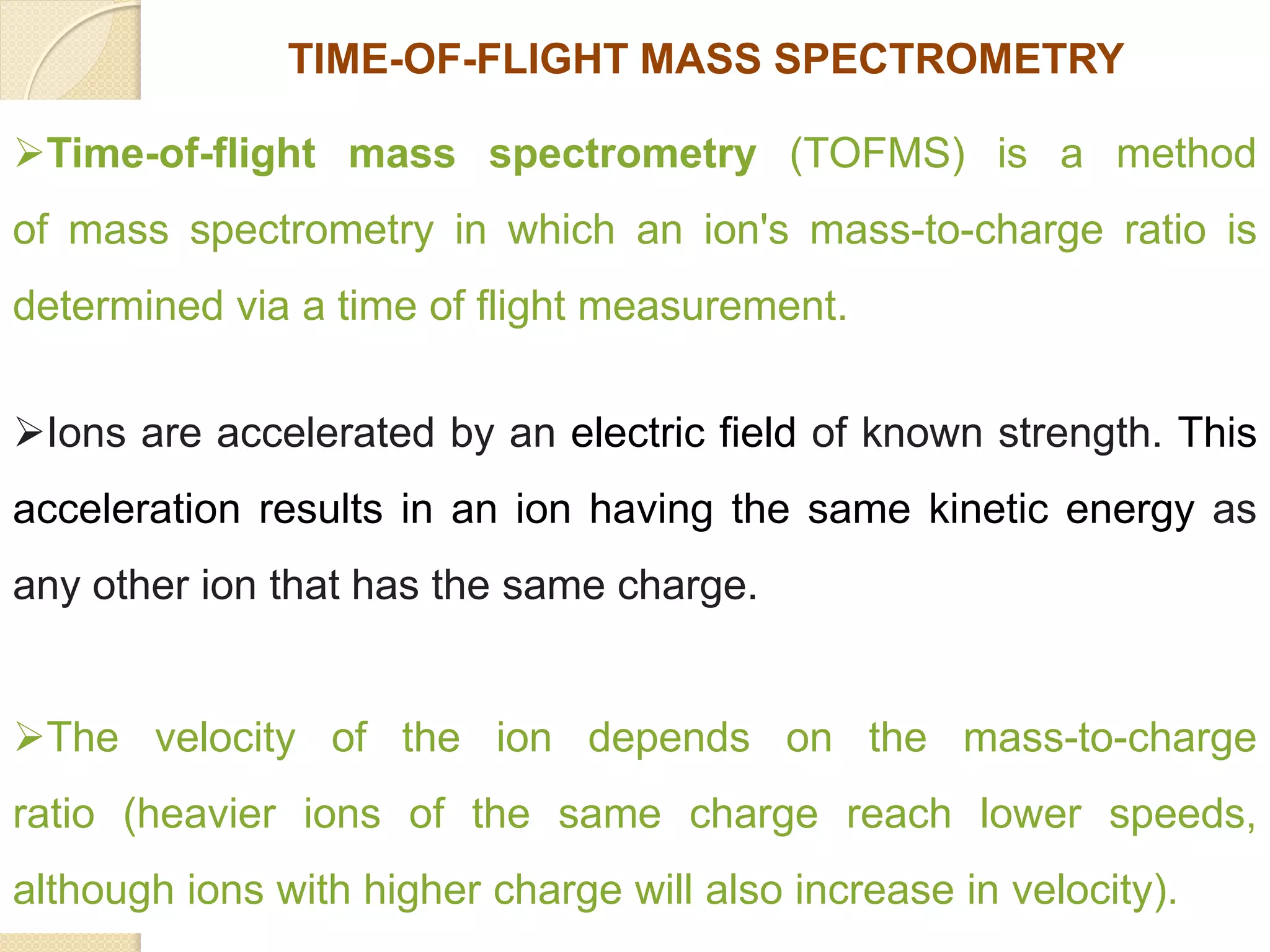
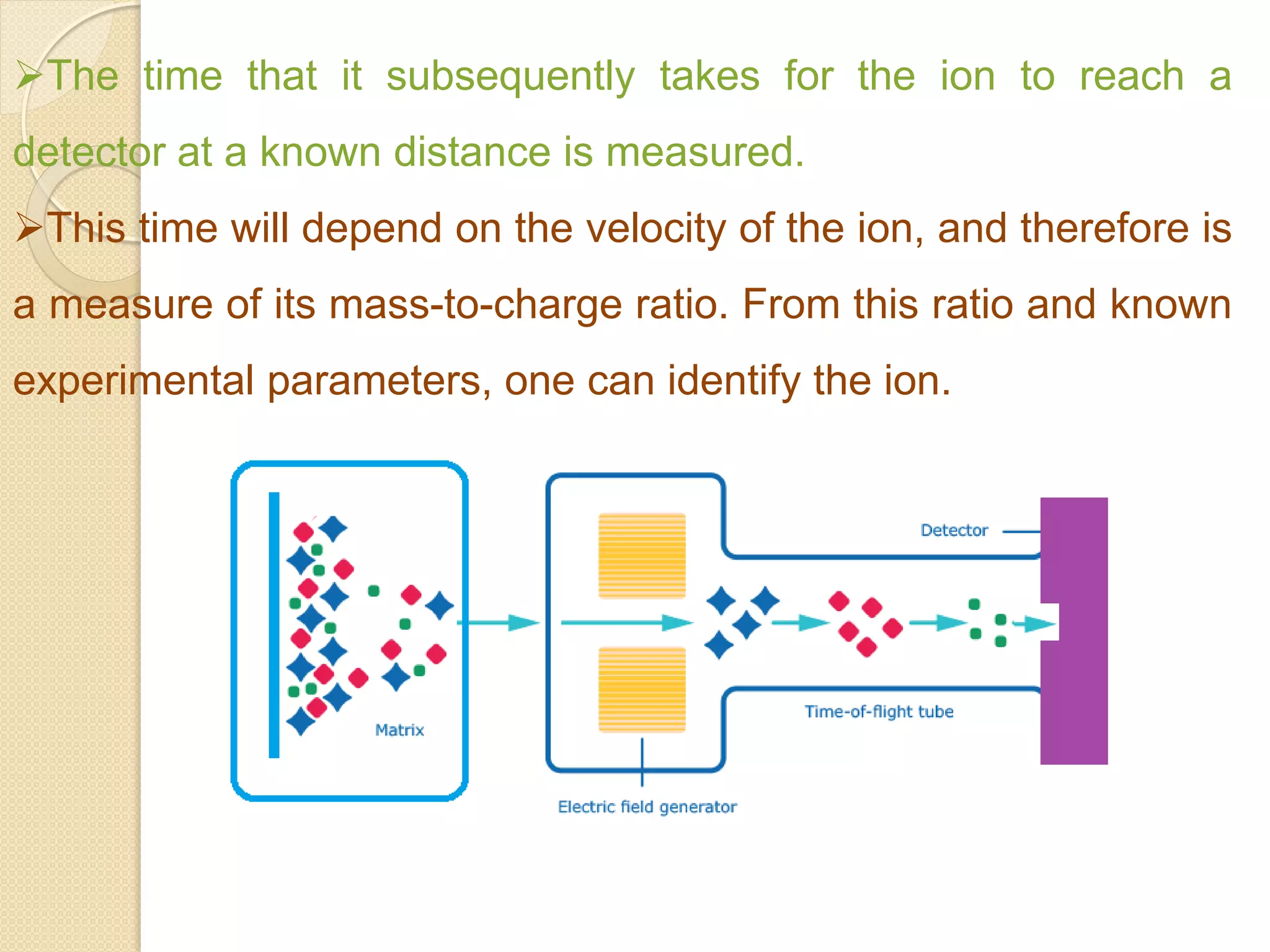
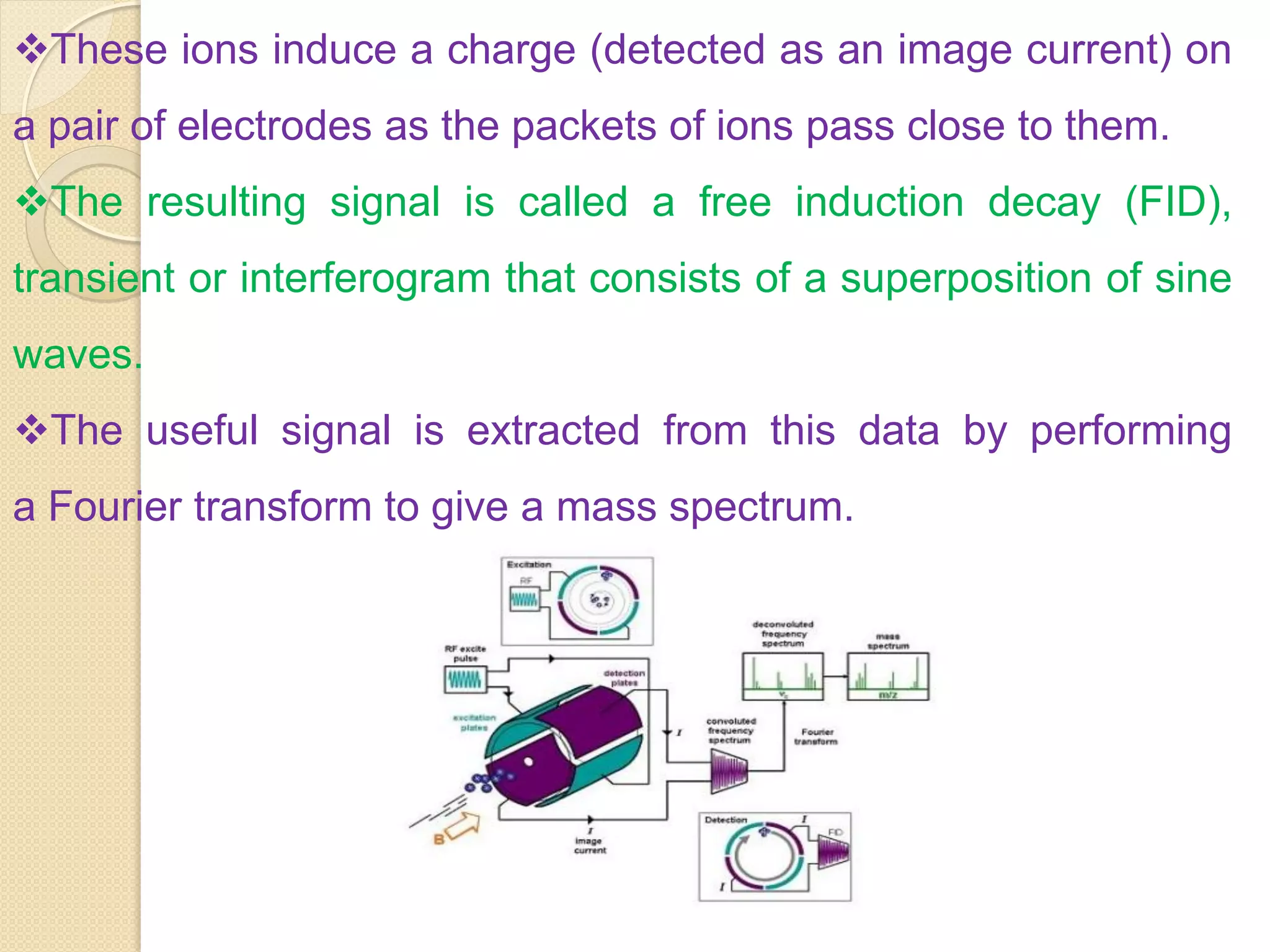
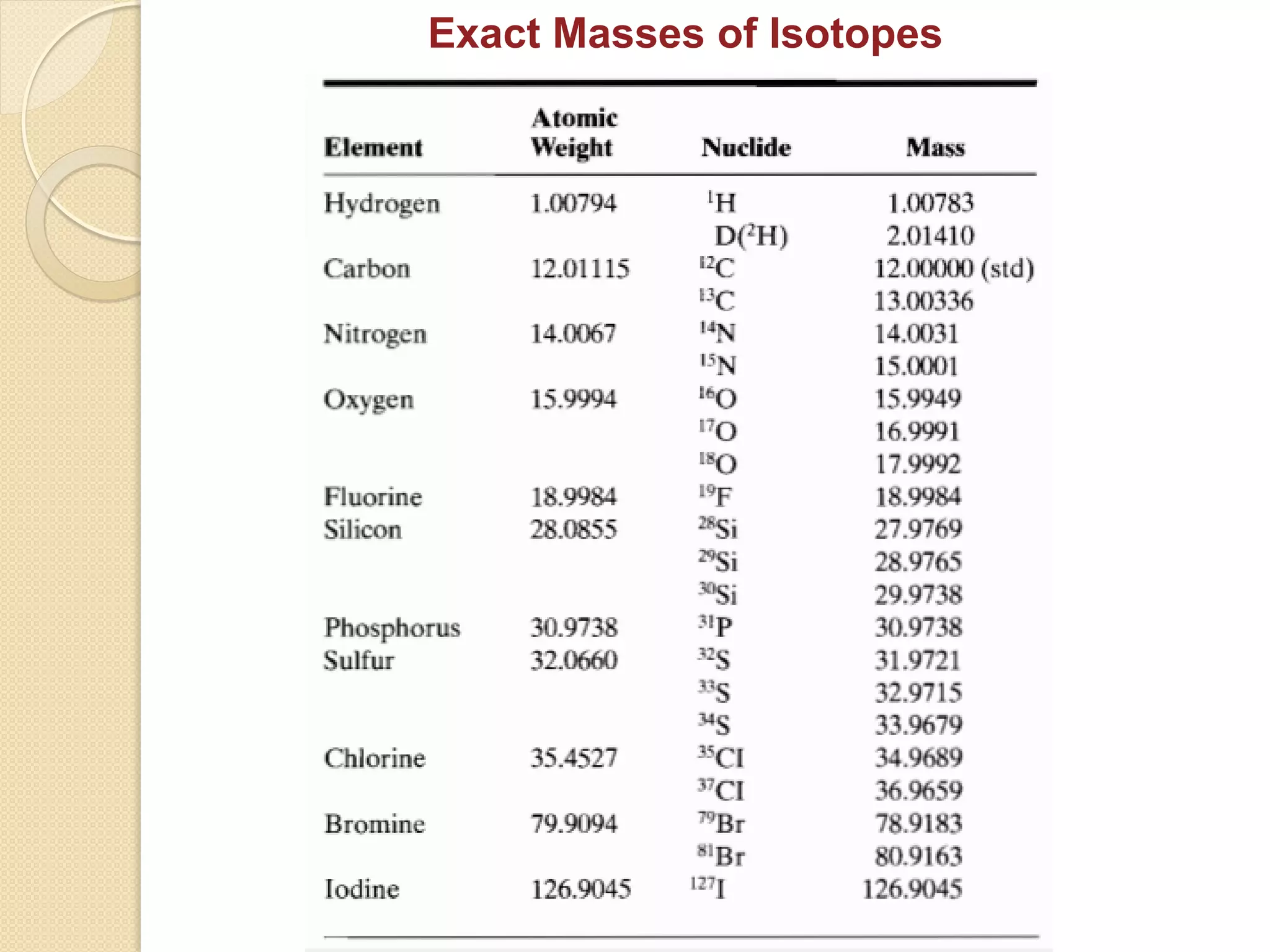
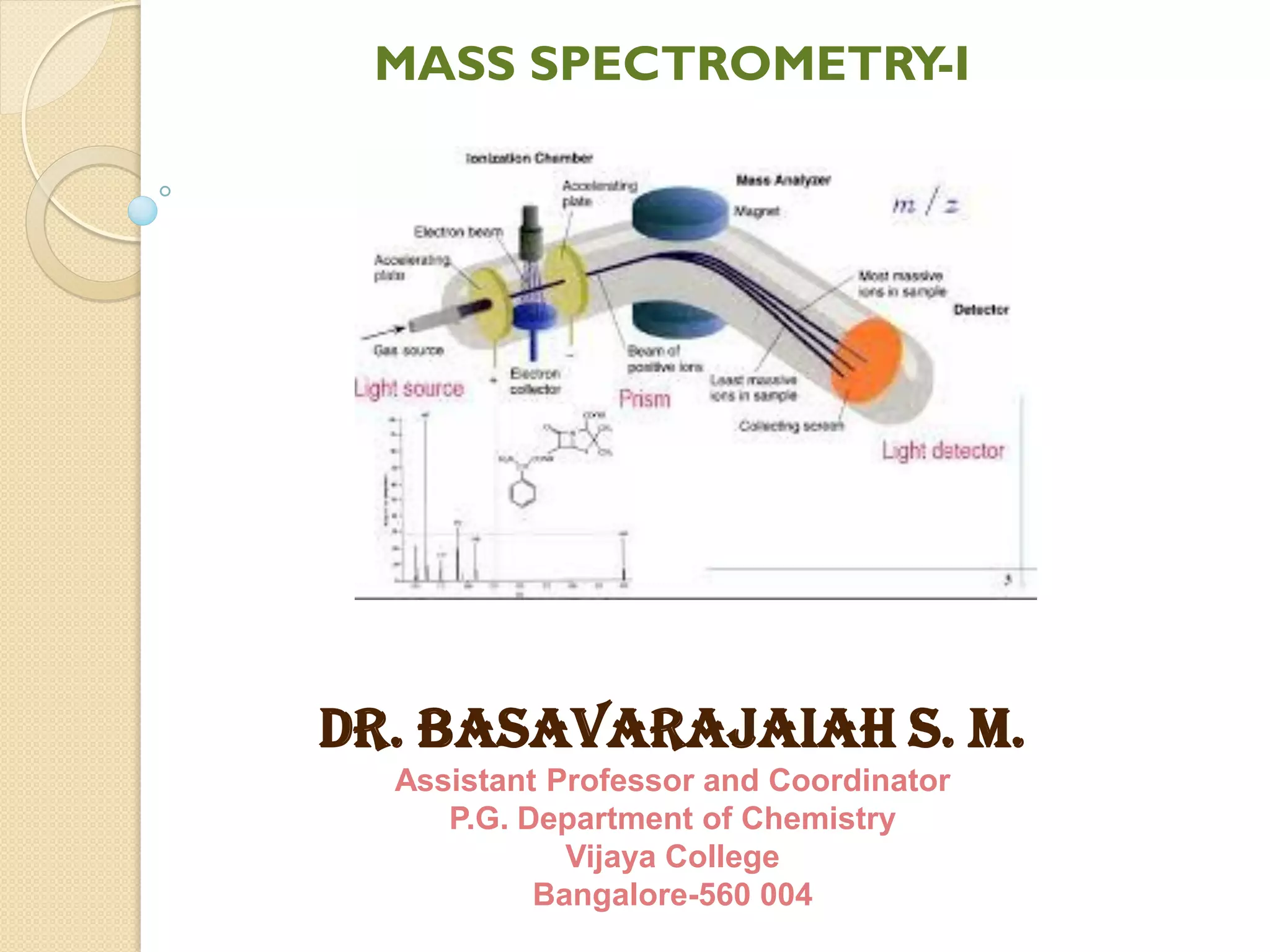
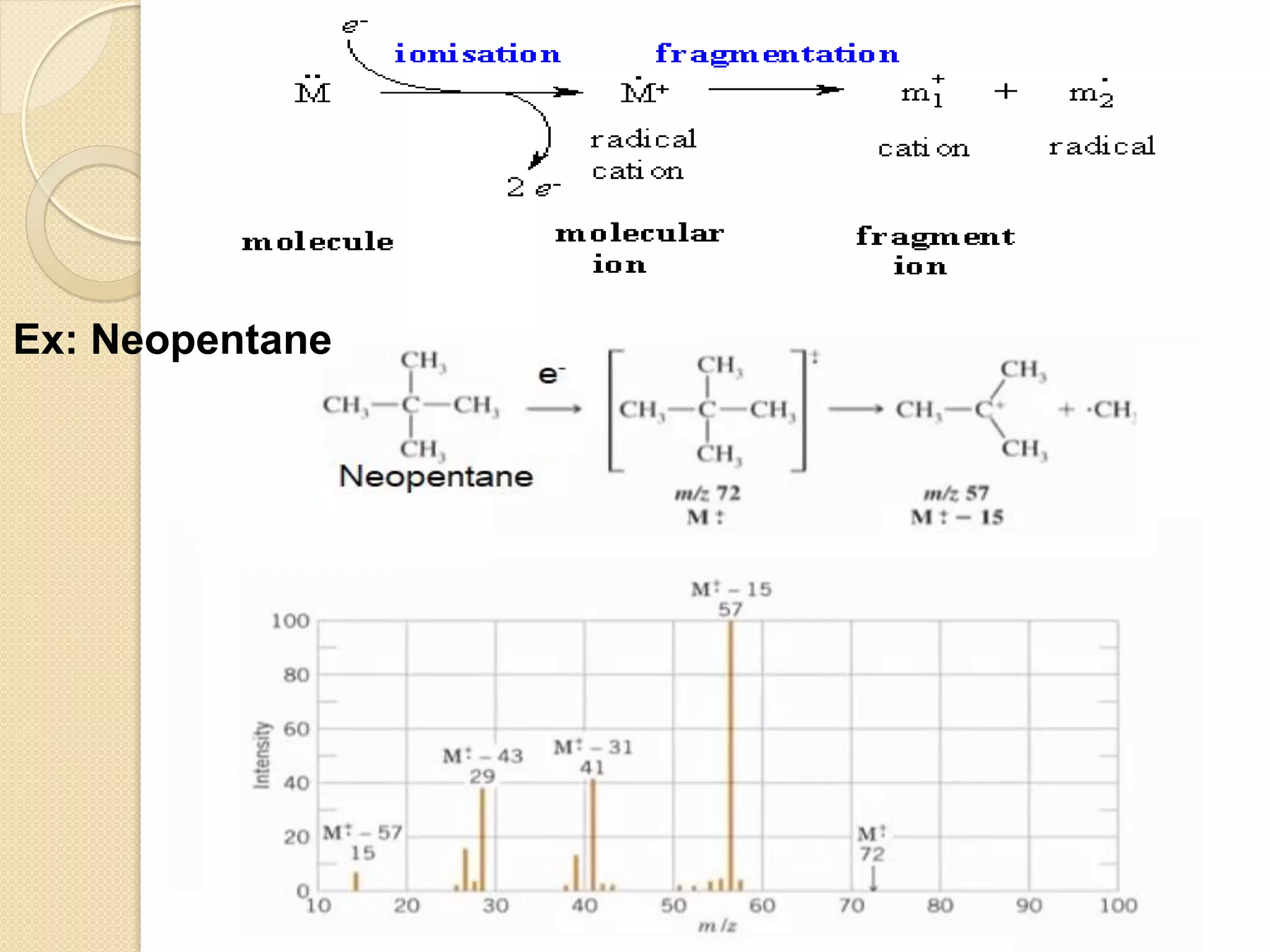
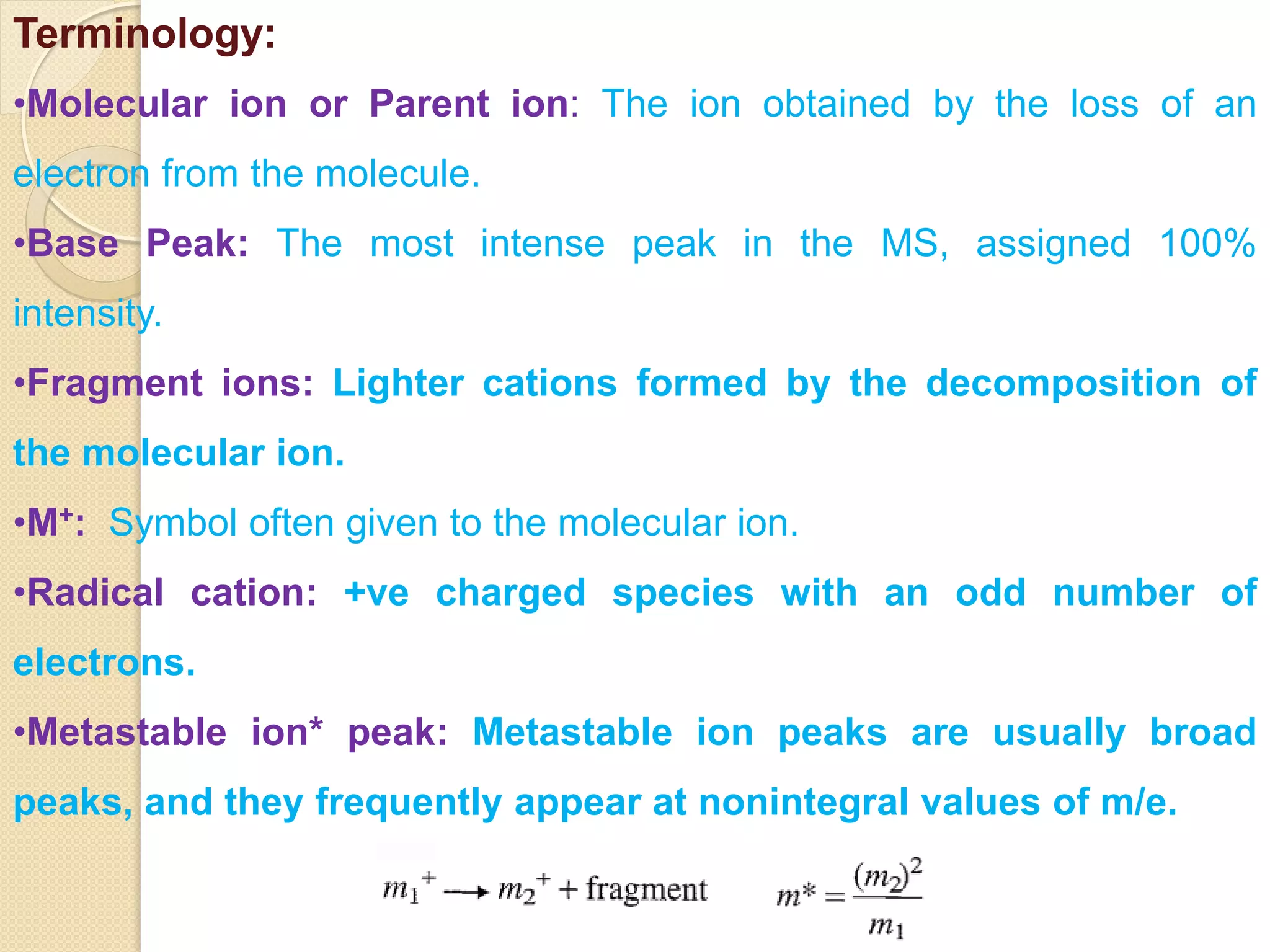
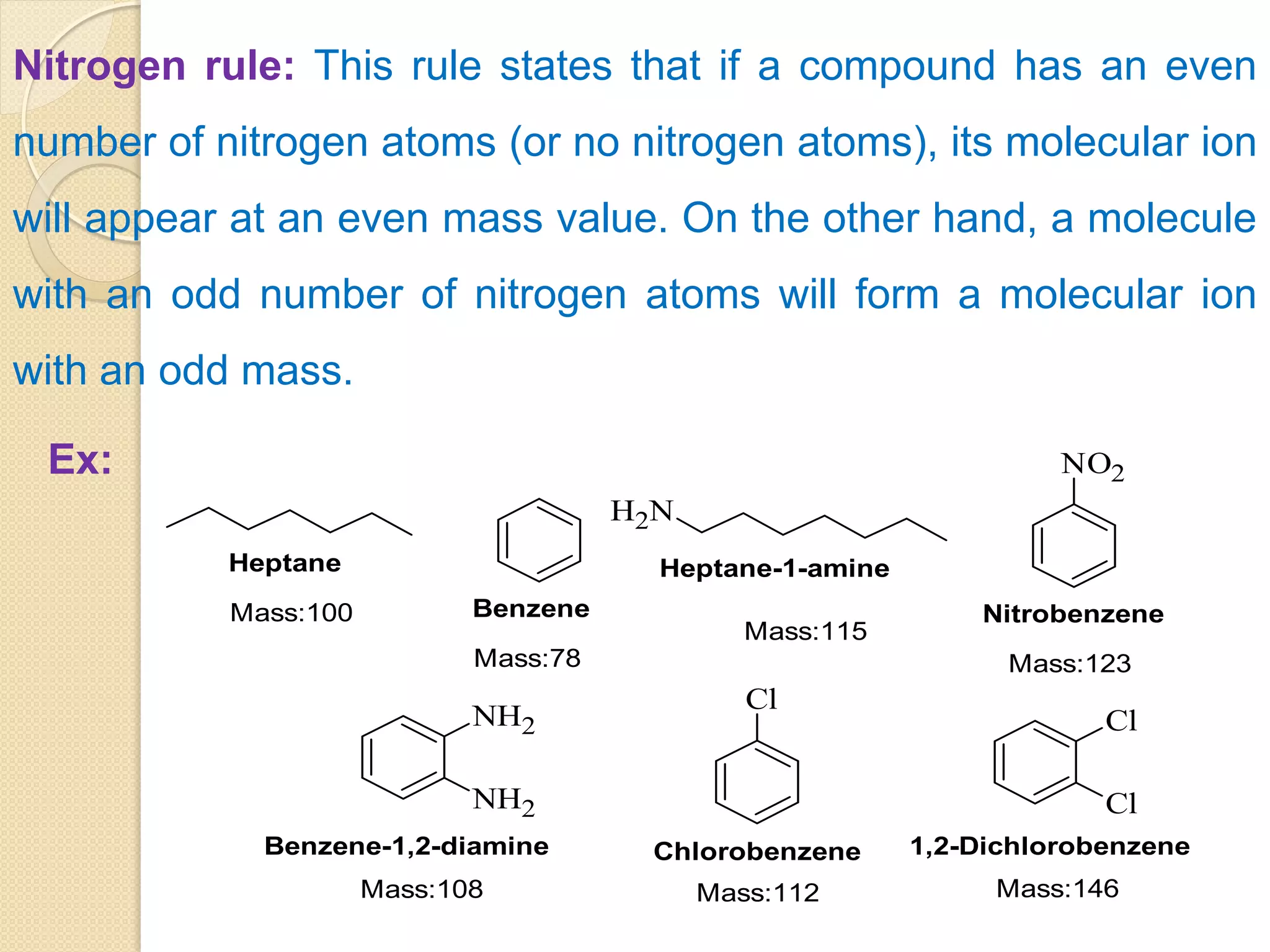
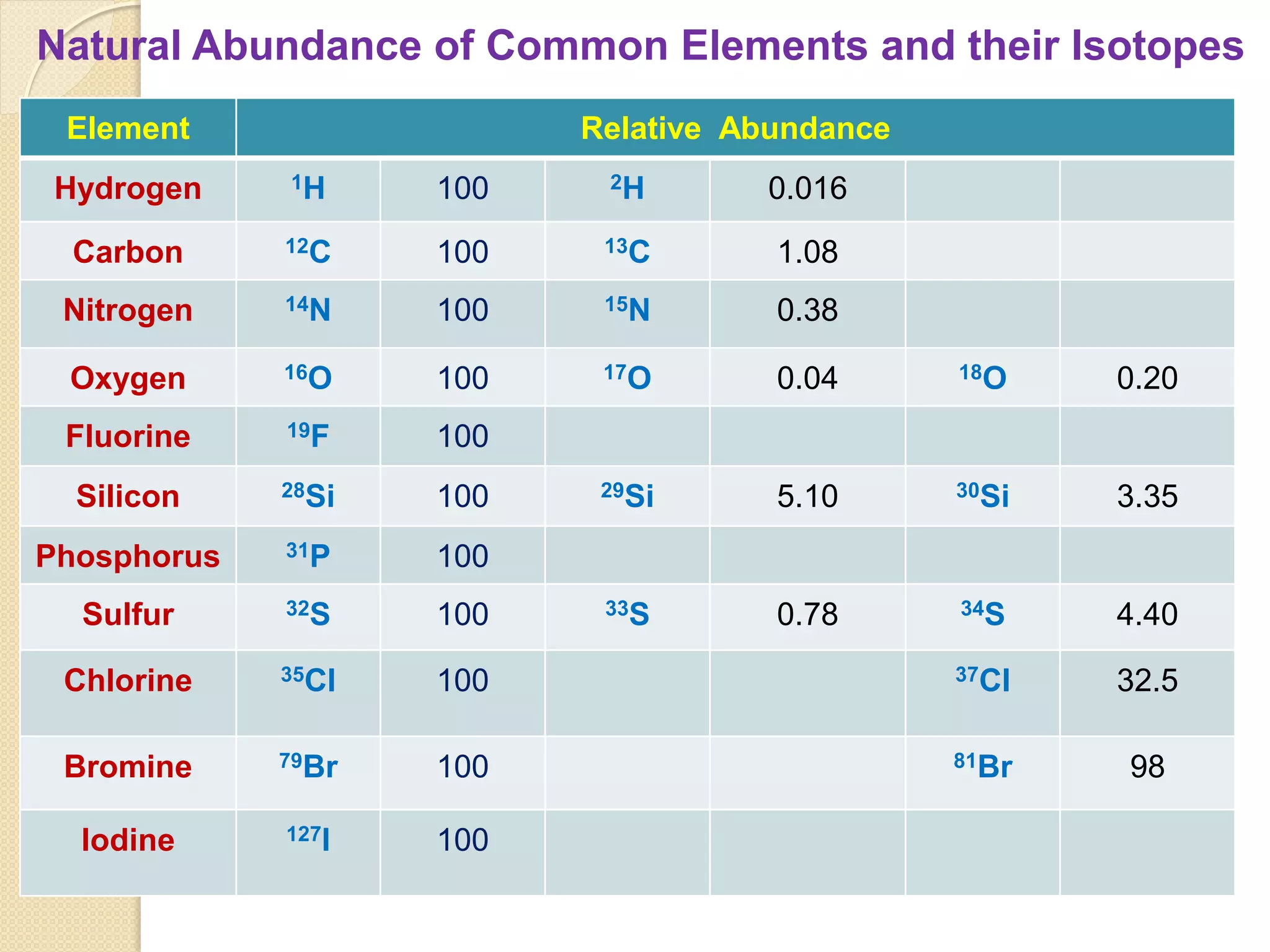
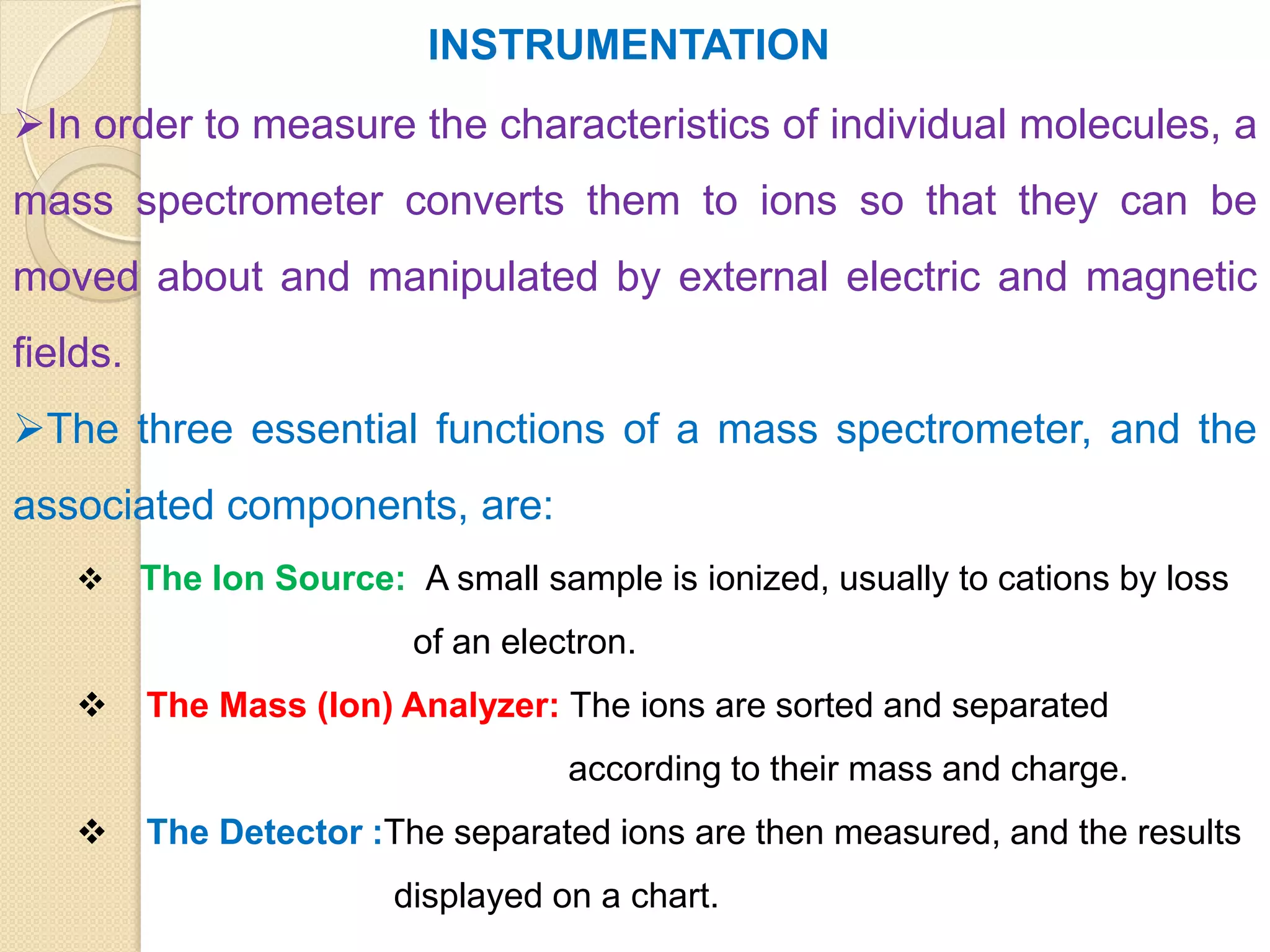
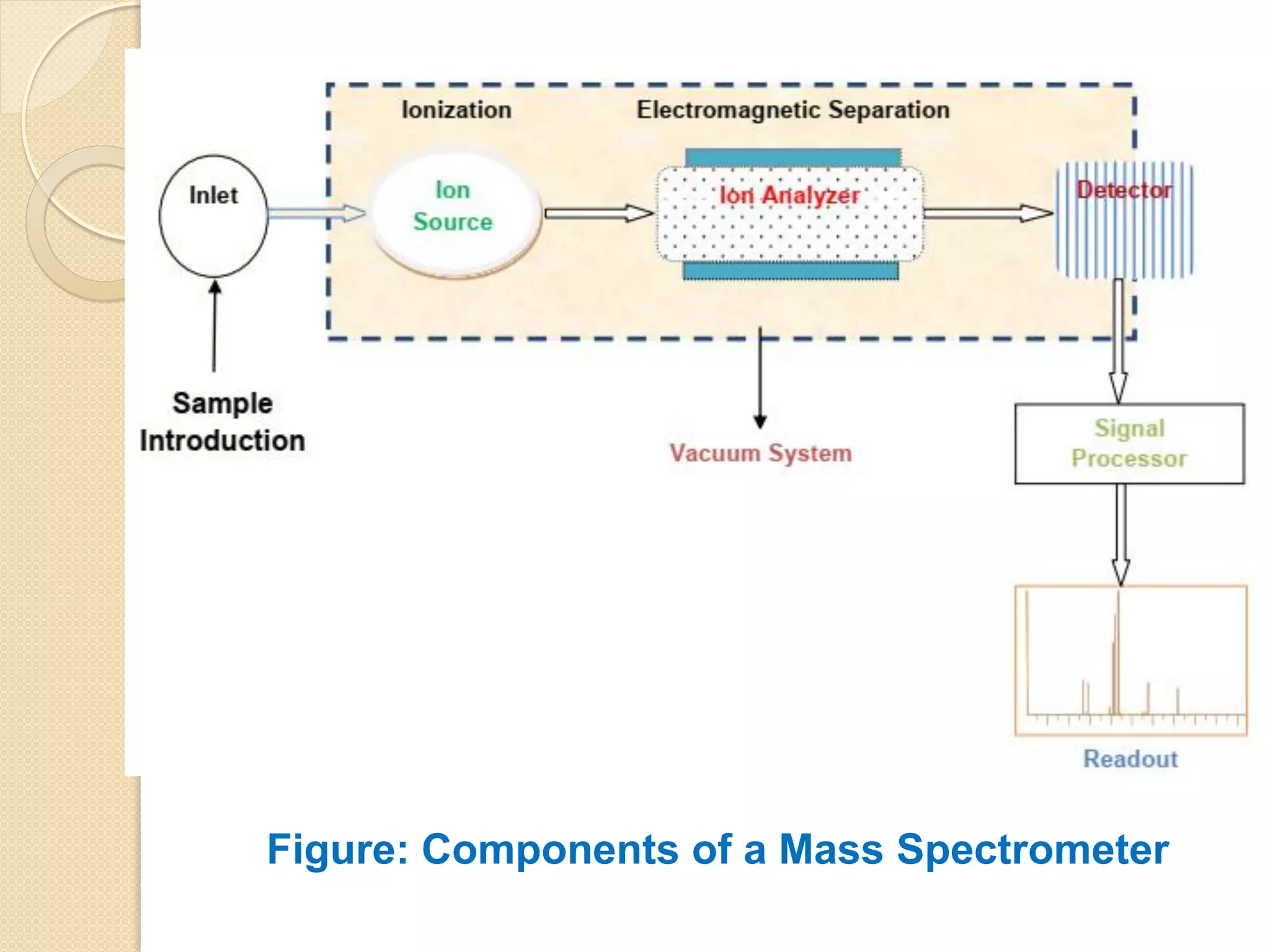
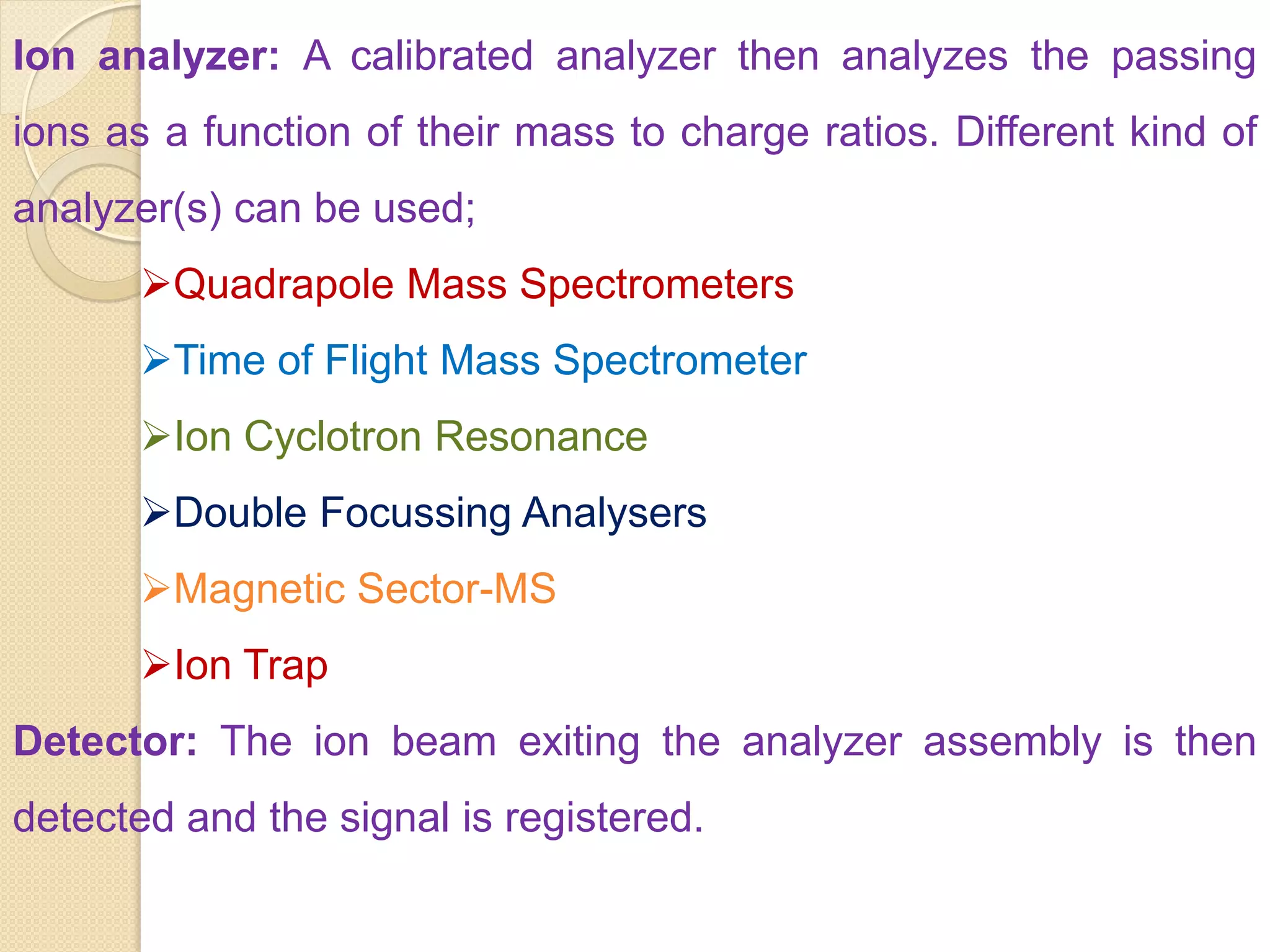
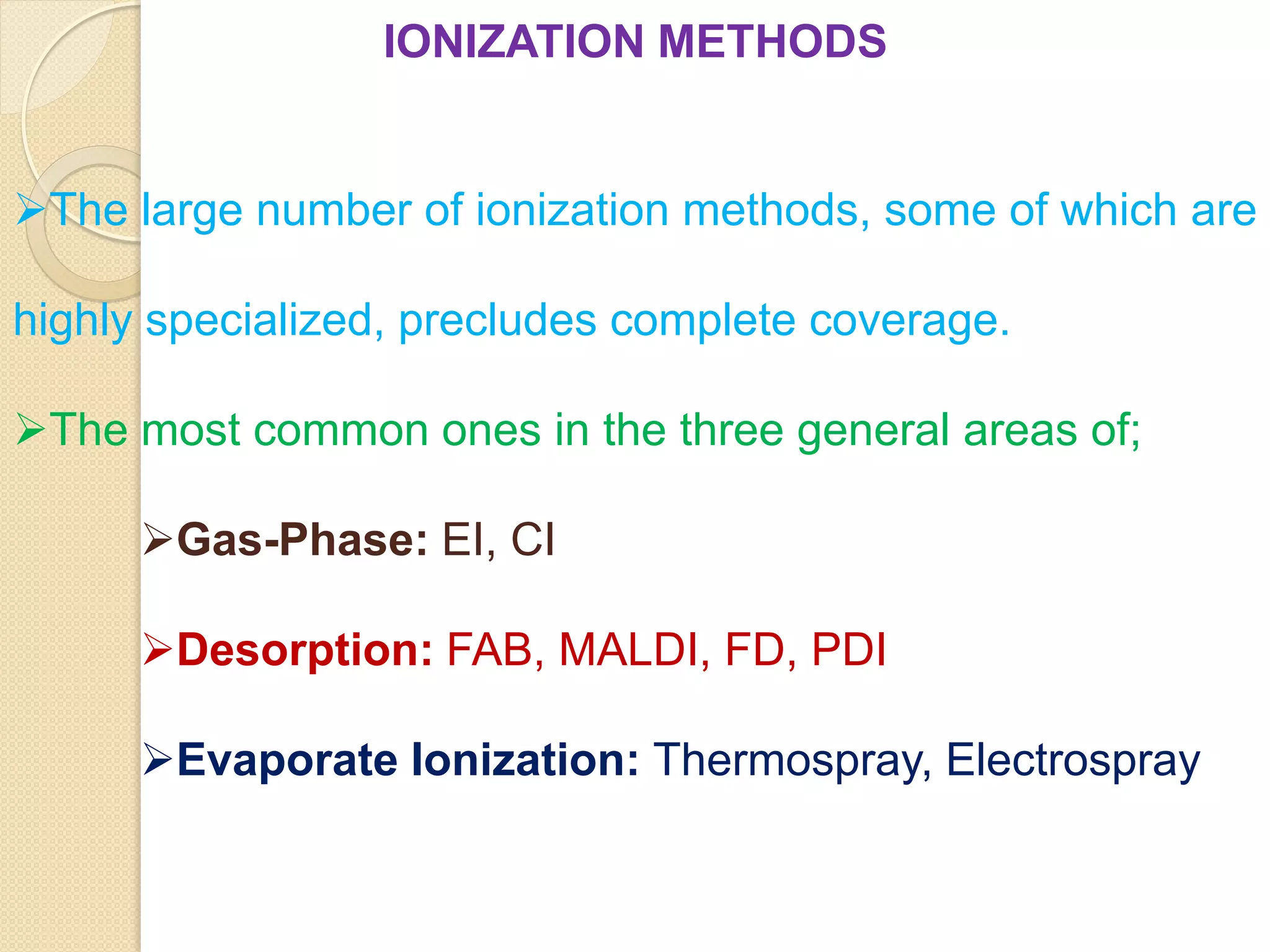
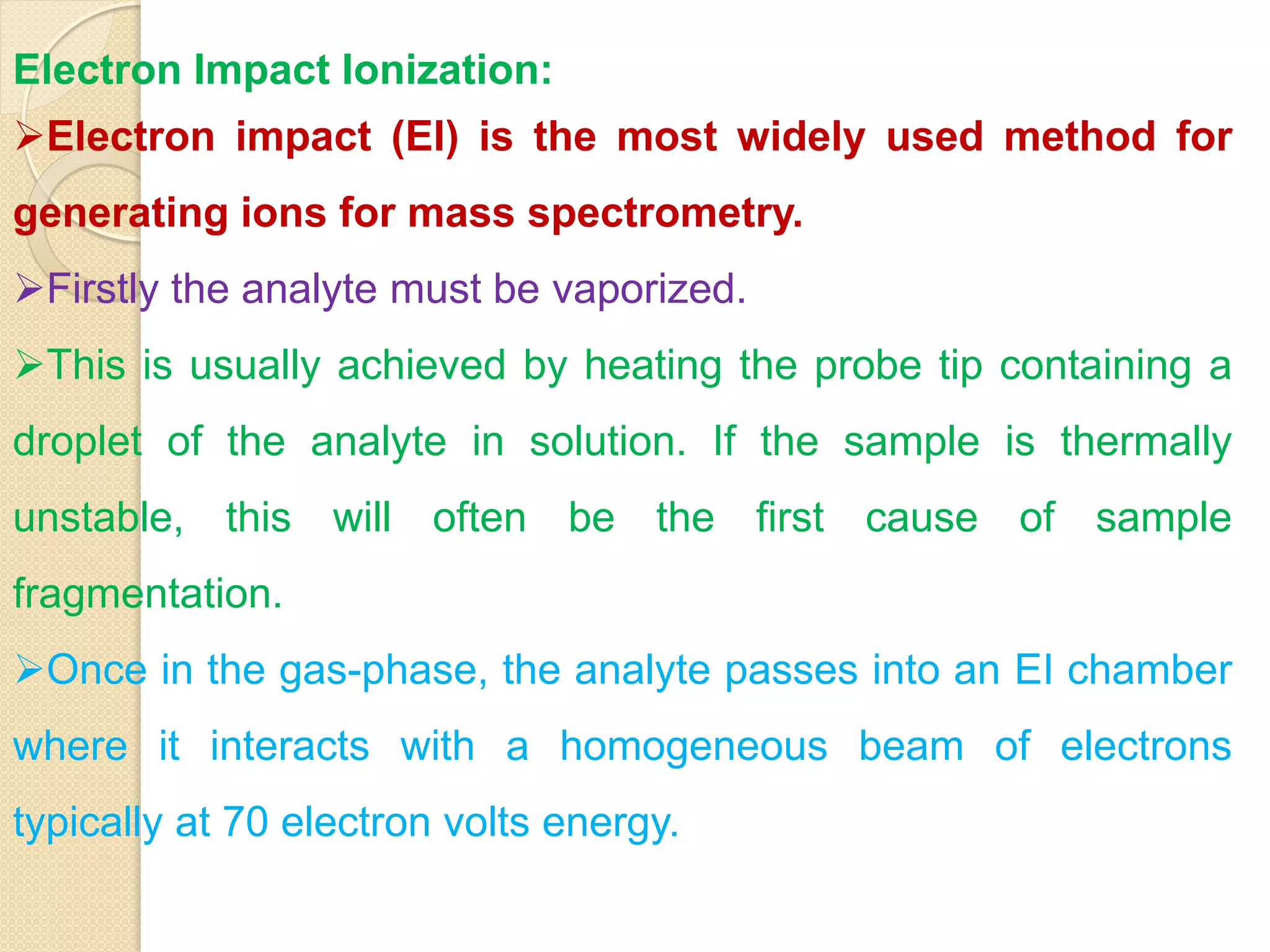
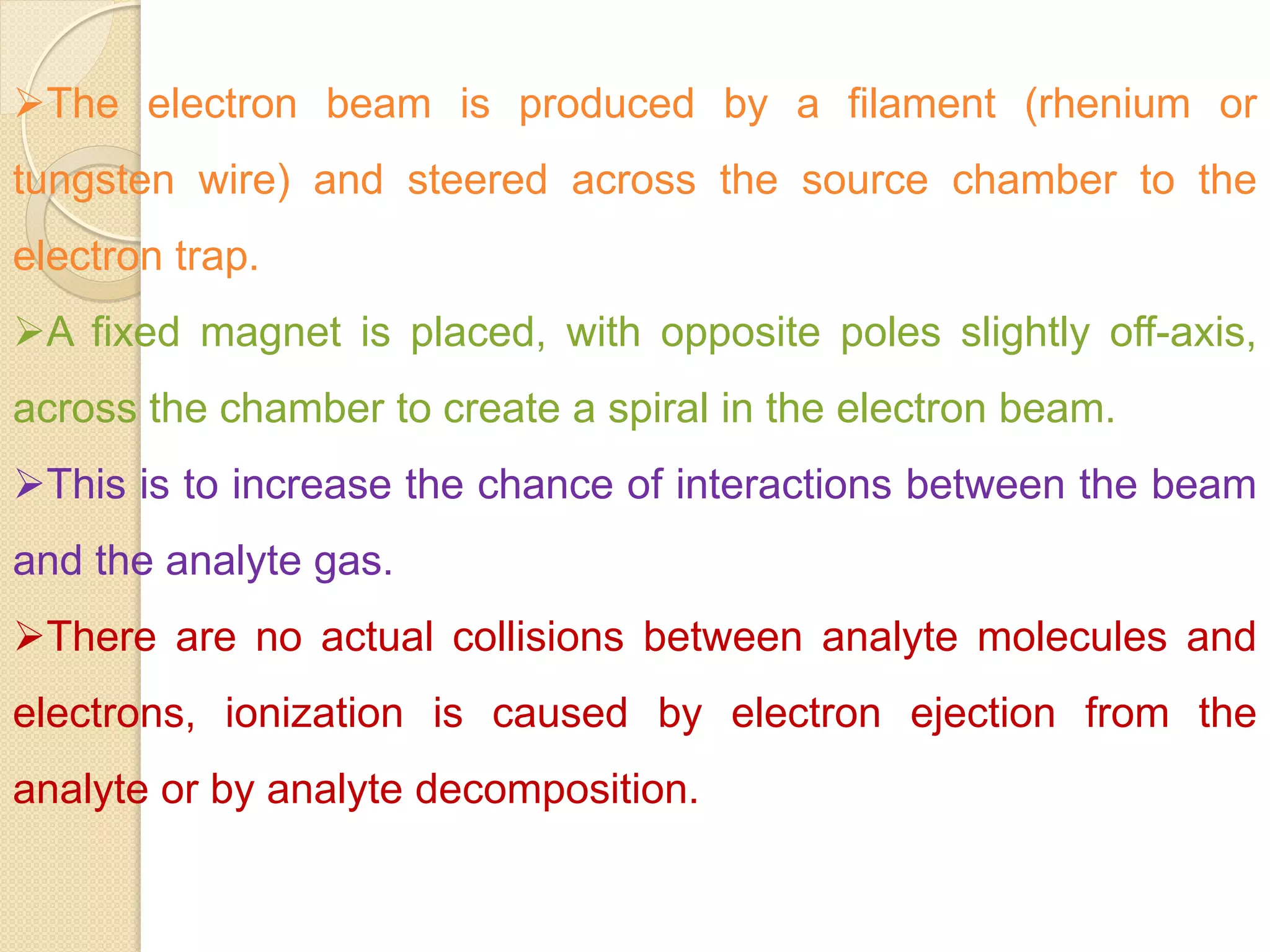
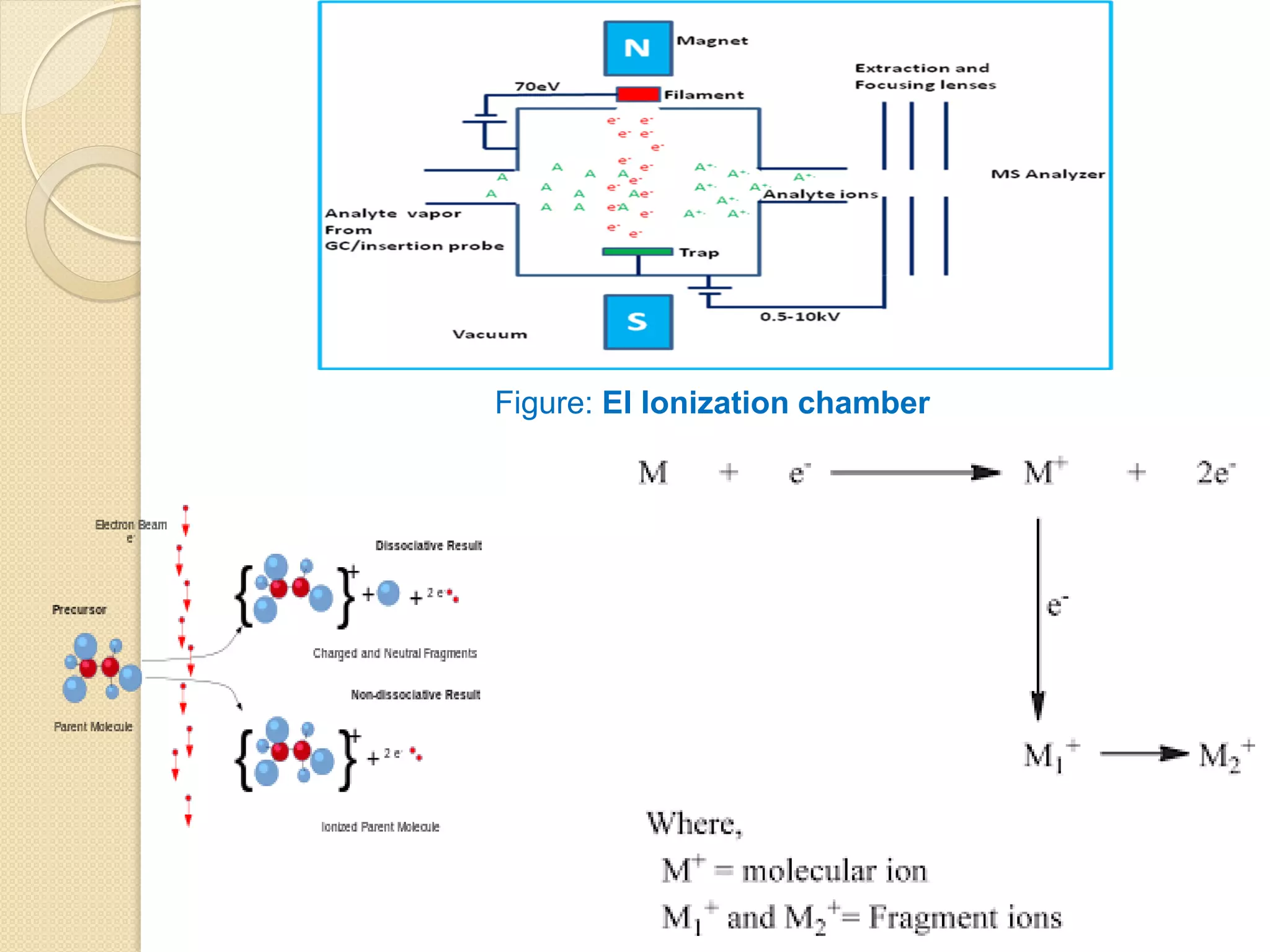
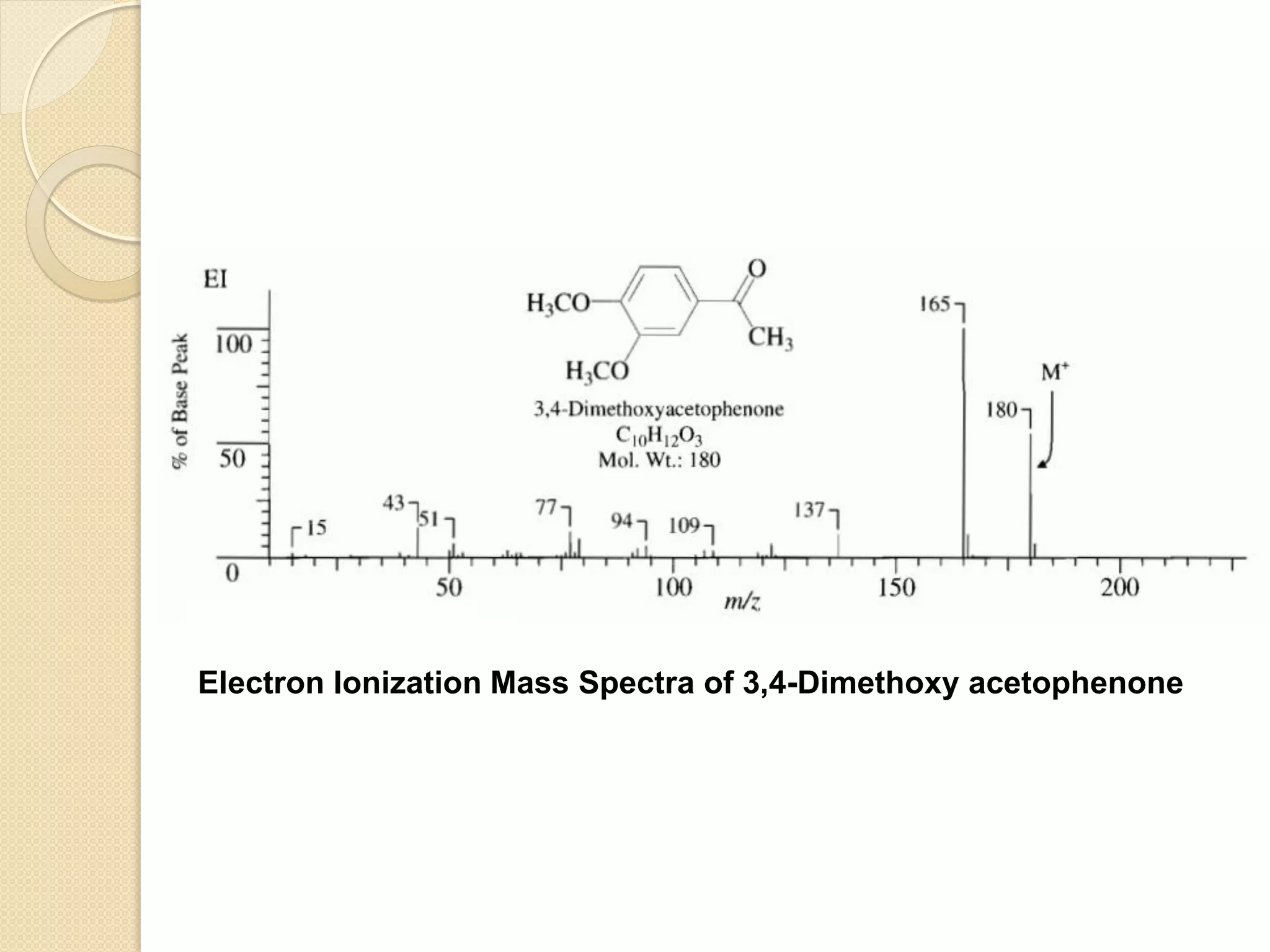
Mass spectrometry is an analytical technique for quantifying known materials, identifying unknown compounds, and elucidating molecular structures through the conversion of samples into gaseous ions characterized by their mass-to-charge ratios. The process involves various ionization methods, instrumentation components like ion sources, mass analyzers, and detectors, and applications across multiple scientific fields. Key techniques include electron impact, chemical ionization, and mass spectrometry variants like MALDI and ESI, which have distinct benefits for analyzing different sample types.


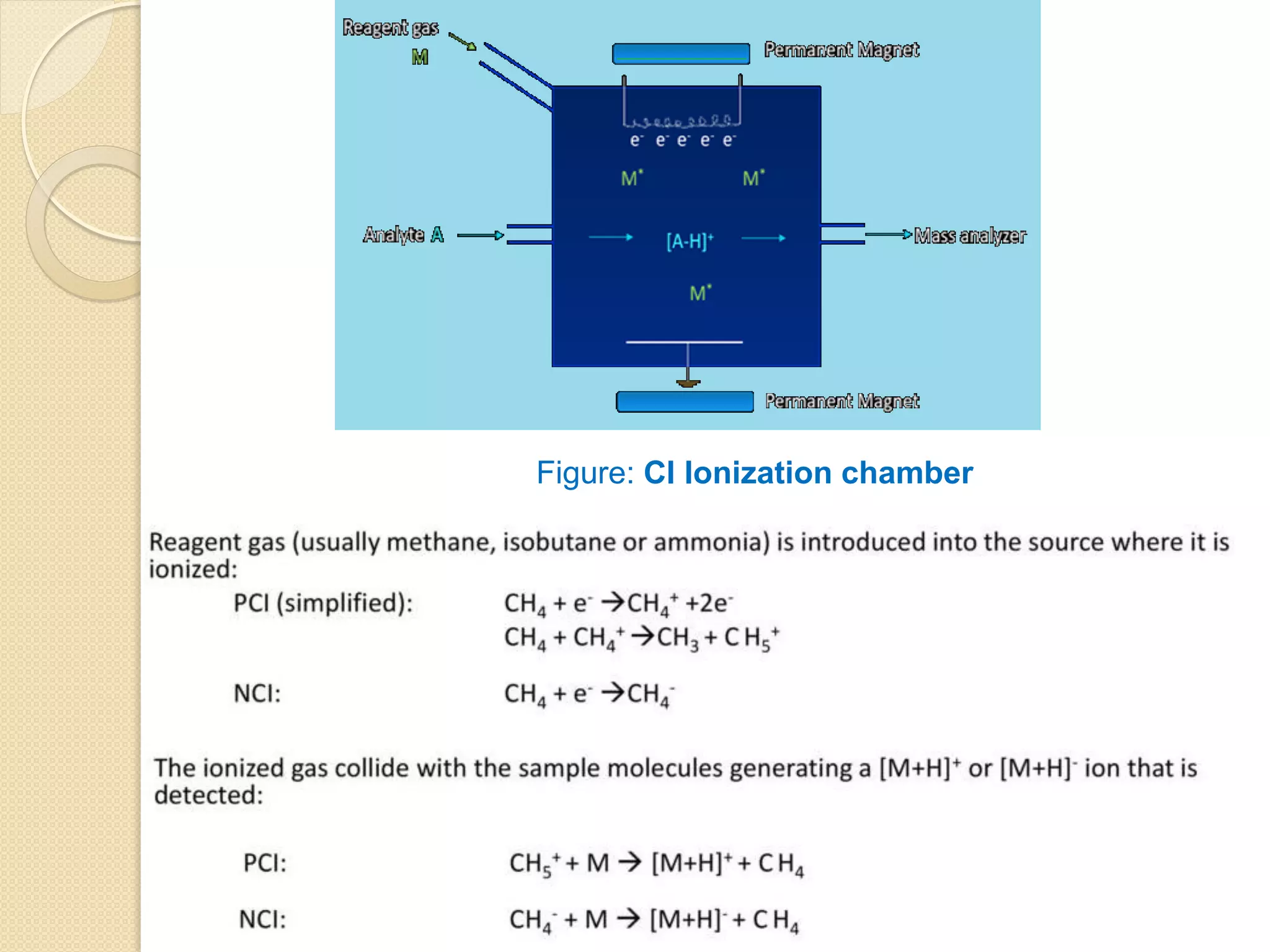
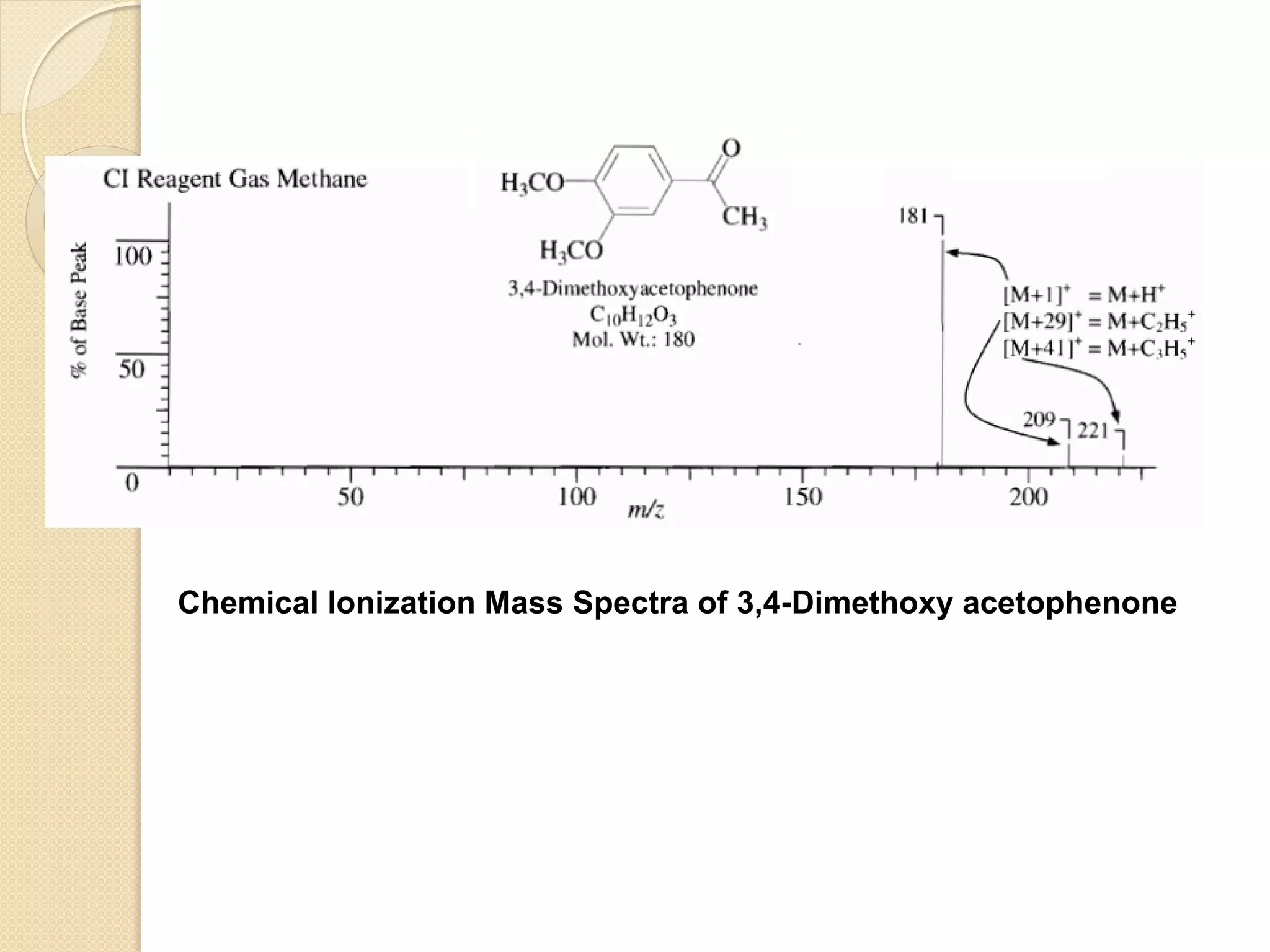
![In CI, ion molecule reactions occur between ionized reagent gas
molecules (G) and volatile analyte neutral molecules (M) to
produce analyte ions. Pseudo-molecular ion MH+ (positive ion
mode) or [M-H]- (negative ion mode) are often observed.
Unlike molecular ions obtained in EI method, MH+ and [M-H]-
detection occurs in high yield and less fragment ions are
observed.
Positive ion mode:
GH+ + M ------> MH+ + G
Negative ion mode:
[G-H]- + M ------> [M-H]- + G](https://image.slidesharecdn.com/massspectrometry-i-201213173915/75/Mass-spectrometry-i-20-2048.jpg)
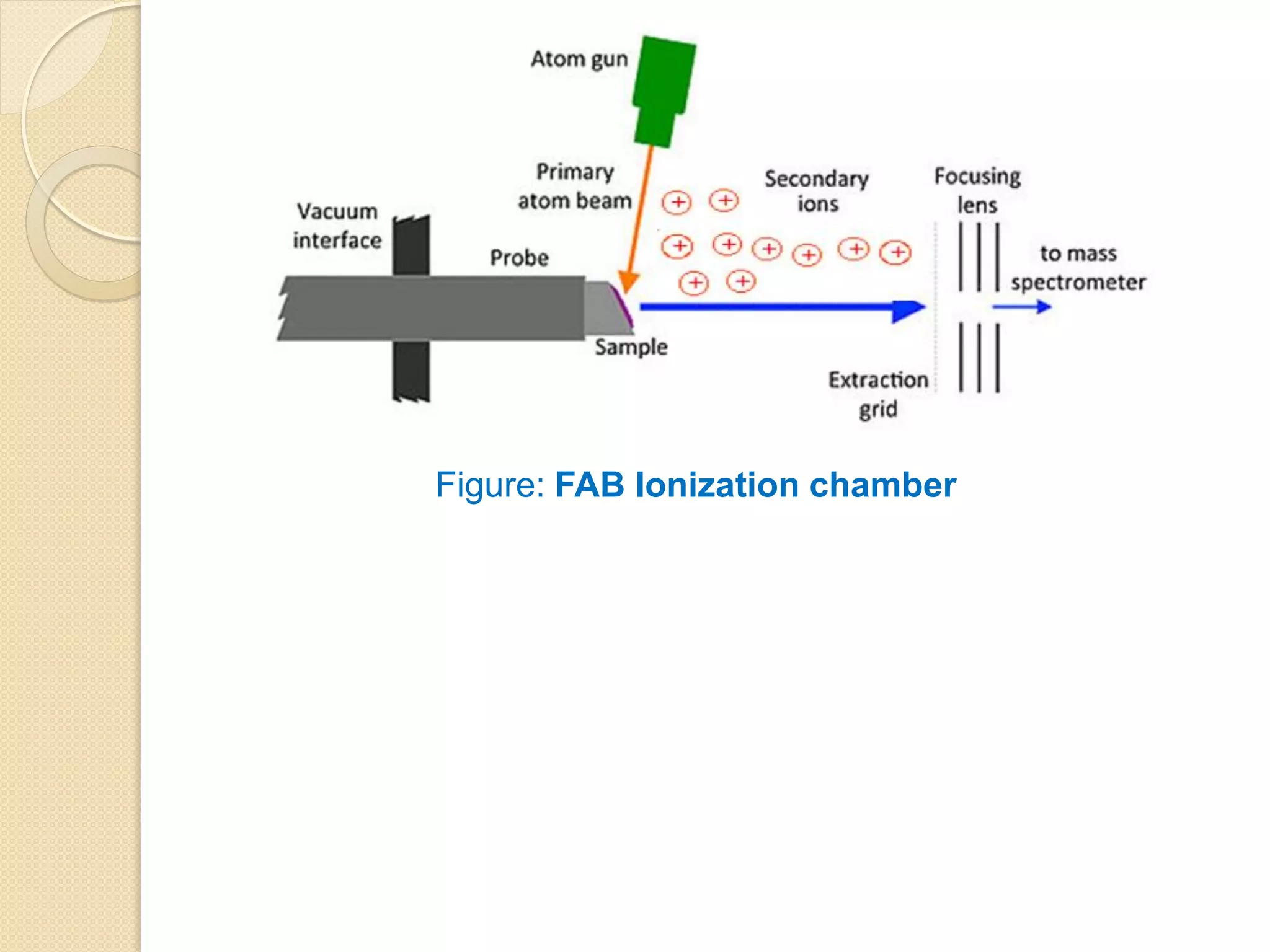
![FAST ATOM BOMBARDMENT IONIZATION
Fast atom bombardment (FAB) uses high-energy Xenon and Argon
atoms (6-10 keV) to bombard samples dissolved in a liquid of low vapor
pressure (Matrix) (e.g., glycerol, m-nitro benzyl alcohol, diethanolamine
etc.).
The matrix protects the sample from excessive radiation damage. A
related method, liquid secondary ionization mass spectrometry. LSIMS,
is similar except that it uses somewhat more energetic cesium ions (10-
30 keV).
In both methods, positive ions (by cation attachment) ([M+1]+ or
[M+Na]+) and negative ions (by deprotonation [M-1]+) are formed; both
types of ions are usually singly charged and, depending on the FAB is
used primarily with large nonvolatile molecules, particularly to determine
molecular weight.](https://image.slidesharecdn.com/massspectrometry-i-201213173915/75/Mass-spectrometry-i-23-2048.jpg)