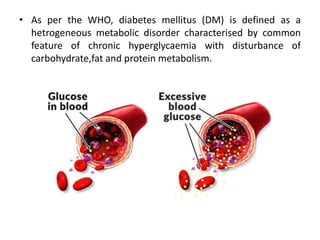
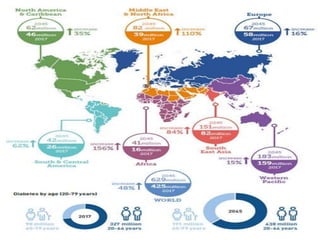
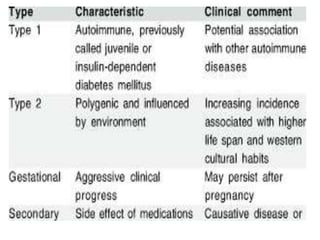
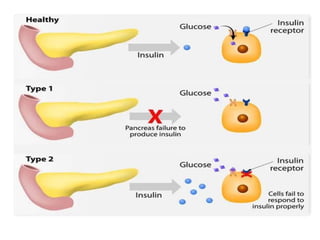
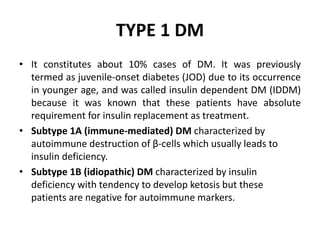
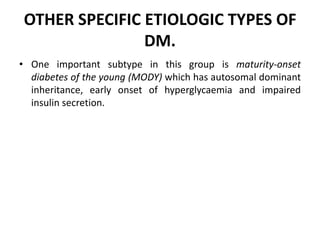
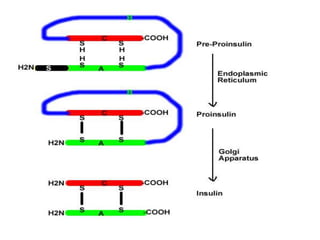
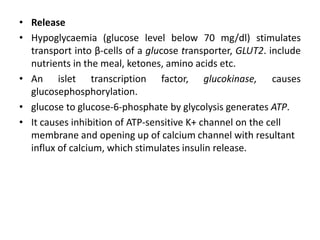
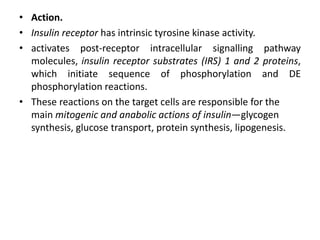
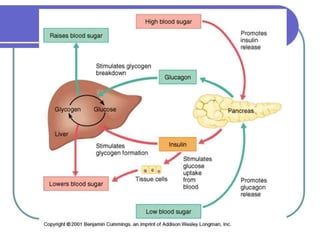
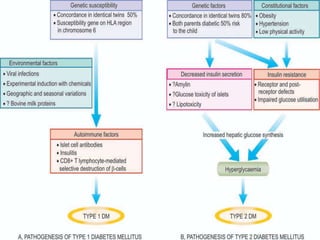
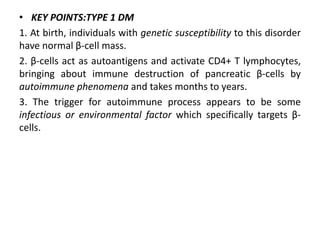
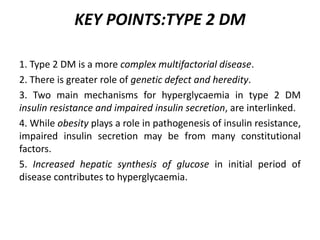
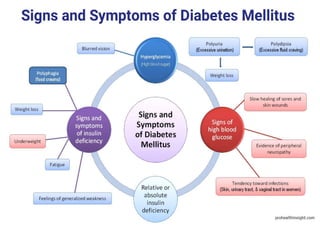
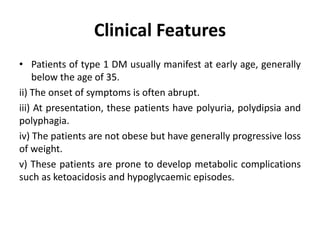
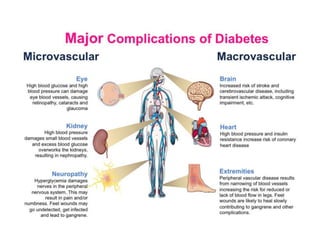
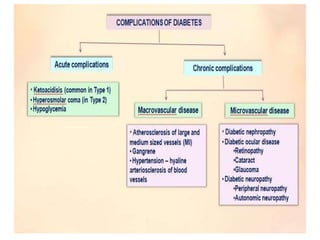
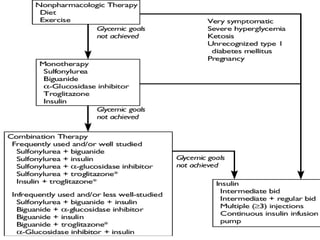
Diabetes mellitus is a metabolic disorder characterized by chronic hyperglycemia. The main types are type 1 diabetes, which accounts for 10% of cases and results from autoimmune destruction of beta cells, and type 2 diabetes, which accounts for 80% of cases and involves insulin resistance and impaired insulin secretion. Diagnosis is confirmed by random plasma glucose over 200 mg/dL or an oral glucose tolerance test. Complications include acute issues like ketoacidosis and hypoglycemia, and chronic complications involving the cardiovascular, renal, neurological, and ophthalmic systems.