This document discusses genotoxicity, which is the study of how chemical and physical agents affect the integrity of genetic material in cells. It covers the history, importance, and regulatory guidelines for conducting genotoxicity tests, including various methodologies and their applications. The content emphasizes the significance of identifying potential human carcinogens and mutagens as part of regulatory requirements for new chemical entities.





![07-11-2017 6
OECD GUIDELINES
• Genetic Toxicology TGs was first published in 1987 .Following a global
update of the Genetic Toxicology TGs [1997,2013,2014,2015,2016]
• Latest revision provides :
(1)general background and historical information on the OECD genetic
toxicology TGs.
(2) a brief overview of the important types of genetic damage evaluated by these
tests.
(3) a description of the specifictests.](https://image.slidesharecdn.com/genotoxicitystudies-200705081255/85/Genotoxicity-studies-6-320.jpg)

![07-11-2017 11
Pre-incubation method
Pre-incubated with the test strain .05-0.1ml (approx. 108 cells) & sterile
buffer or the metabolic activation system (s9 0.5 ml) usually for 20 min
@30-37°c [aeration+ shaker – 48 to 72 hrs]
Mix overlay agar (2ml) and pouring onto the surface of a minimal agar
plate
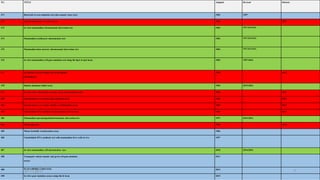
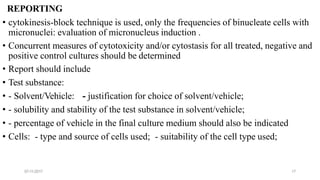
REPORT
• number of revertant colonies per plate ( with +ve & -ve coloies nos)
• Standard deviation](https://image.slidesharecdn.com/genotoxicitystudies-200705081255/85/Genotoxicity-studies-11-320.jpg)

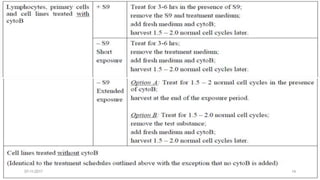

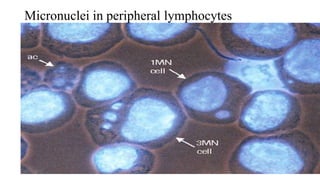
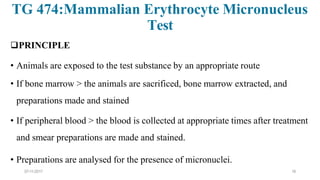

![07-11-2017 20
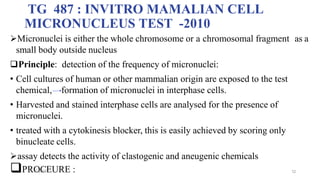
• Bone marrow cells are usually obtained from the femurs or tibias immediately
after sacrifice , and stained using established methods.
• Blood :tail vein or other appropriate blood vessel , smear preparations are
made and then stained
• DNA specific stain [e.g. acridine orange or Hoechst 33258 plus pyronin-Y]
• Analysis
• at least 200 erythrocytes
• At least 2000 immature erythrocytes per animal are scored for the incidence of
micronucleated immature erythrocytes.
• Result: presented in tabular form , dose-related increase ,
• Statistical methods may be used : statistical significants](https://image.slidesharecdn.com/genotoxicitystudies-200705081255/85/Genotoxicity-studies-20-320.jpg)

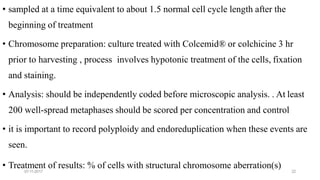


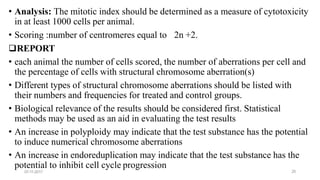

![07-11-2017 27
• A repeat dose treatment regimen can be used, such as in conjunction with a test on
another endpoint that uses a 28 day administration period.
• metaphase arresting chemical injected intraperitoneally , euthanasia , Animals are
sampled at an appropriate interval thereafter.
• The highest dose may also be defined as a dose that produces some indication of
toxicity in the spermatogonial cells
• Observations: General clinical observations , once a day. health condition , morbidity
and mortality
• Chromosome preparation: After euthanasia, cell suspensions are obtained from
testes,(hypotonic solution ) .The cells spread on slides and stained [blind coded ].
• Analysis :200 well spread metaphases should be scored , Chromosome and chromatid-
type aberrations should be recorded separately and classified](https://image.slidesharecdn.com/genotoxicitystudies-200705081255/85/Genotoxicity-studies-27-320.jpg)


![07-11-2017 31
REFERENCE
1. Essential concepts in toxicology by prof.Dr.Gupta , page no. 139-151
2. Importance of Genotoxicity & S2A guidelines for genotoxicity testing for pharmaceuticals
by Shaily Umang Shah. IOSR Journal of Pharmacy and Biological Sciences (IOSRJPBS)
ISSN : 2278-3008 Volume 1, Issue 2 (May-June 2012), PP 43-54 www.iosrjournals.org.
3. Requirements And Guidelines For Permission To Import And / Or Manufacture Of New
Drugs For Sale Or To Undertake Clinical Trials. Schedule Y(ammended version) – CDSCO
[rules 122A, 122B, 122D, 122DA, 122DAA and 122E] .
file:///G|/RGCB%20IHEC/Schedule%20Y(ammended%20version)%20-
%20CDSCO.htm[08-05-2014 10:53:51]
4. OECD website , www.oecd.org , genotoxicity test guidelines :29 july 2016
OECD Test guidelines; 471,473,474,475,483,487
• Karthik Yamjala, Meyyanathan Subramania Nainar, Nageswara Rao Ramisetti. Ultra high performance liquid
chromatography with tandem mass spectrometry screening and mutagenicity evaluation of photodegradation
products of Carmoisine (E122) dye in a beverage. Journal of Separation Science. 2016; 39:2246-51.](https://image.slidesharecdn.com/genotoxicitystudies-200705081255/85/Genotoxicity-studies-31-320.jpg)