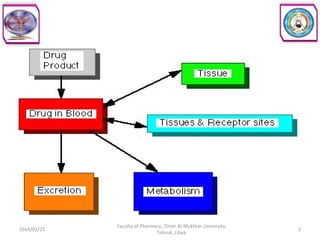
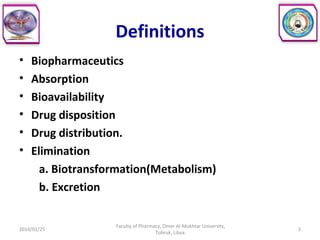
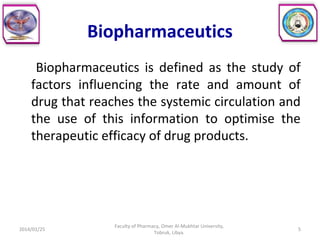
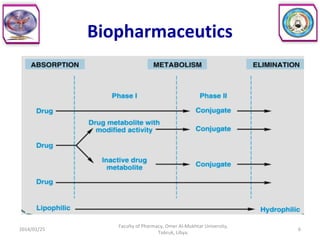
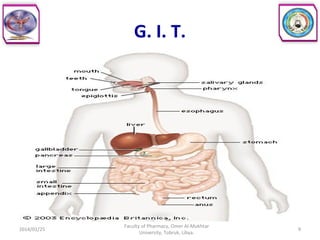
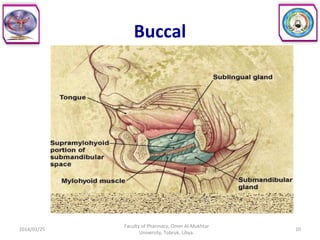
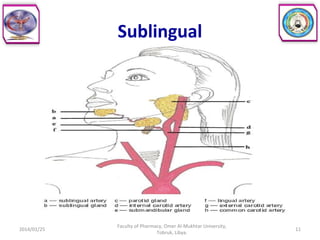
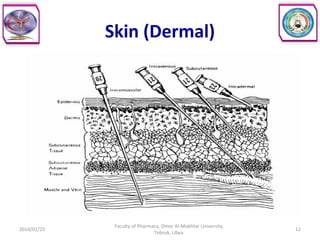
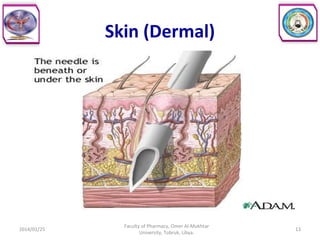
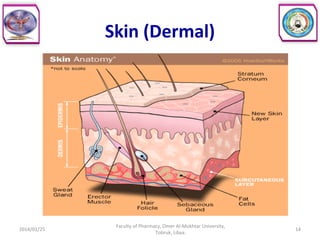
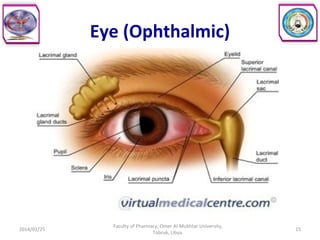
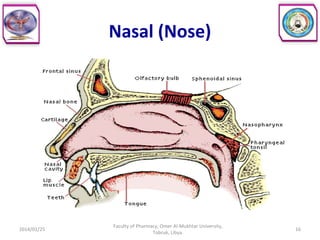
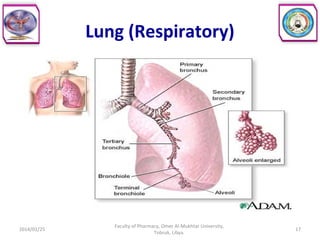
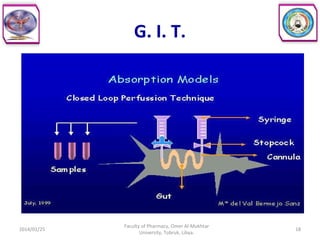
The document defines key concepts in biopharmaceutics including absorption, bioavailability, drug disposition, and elimination. It also defines related terms like pharmacokinetics, pharmacodynamics, and various types of equivalence. The concepts are explained with examples and factors affecting absorption via different routes of administration are discussed. The document also describes the four phases of drug administration and therapy: the pharmaceutical, pharmacokinetic, pharmacodynamic, and therapeutic phases.