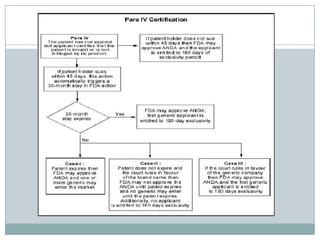
The document discusses the Hatch-Waxman Act and its role in facilitating generic drug approvals in the US. It introduced the Abbreviated New Drug Application (ANDA) process which allows generics to piggyback on the clinical trial data of branded drugs. The Act also established the Orange Book database which lists drug patents. Generic companies can file ANDAs with certifications (Paras I-IV) regarding the patents. Para IV filings are the most complex and involve claims of non-infringement or patent invalidity, often leading to litigation between generic and branded companies. The Act aimed to balance increased generic competition with incentives for continued drug innovation.










![FOUR POSSIBLE CERTIFICATIONS[PARAS]The generic approval process is called Abbreviated New drug Application (ANDA). While filing an ANDA, the generic company has to choose one of the following four options (referred to as paras) A Para I filing is made when the innovator has not made the required patent information in the Orange Book. A Para II filing for the launch of a generic drug is made when the drug is already off patent. A Para III filing is made when the patent for the product exists but the generic company wants to enter the markets after the date of patent expiry passes. A Para IV filing is made when the ANDA applicant believes its product or the use of its product does not infringe on the innovator's patents listed in the Orange Book or where the applicant believes such patents are not valid or enforceable.](https://image.slidesharecdn.com/paraiivorangebook-111007073056-phpapp01/85/Para-i-iv-orange-book-11-320.jpg)