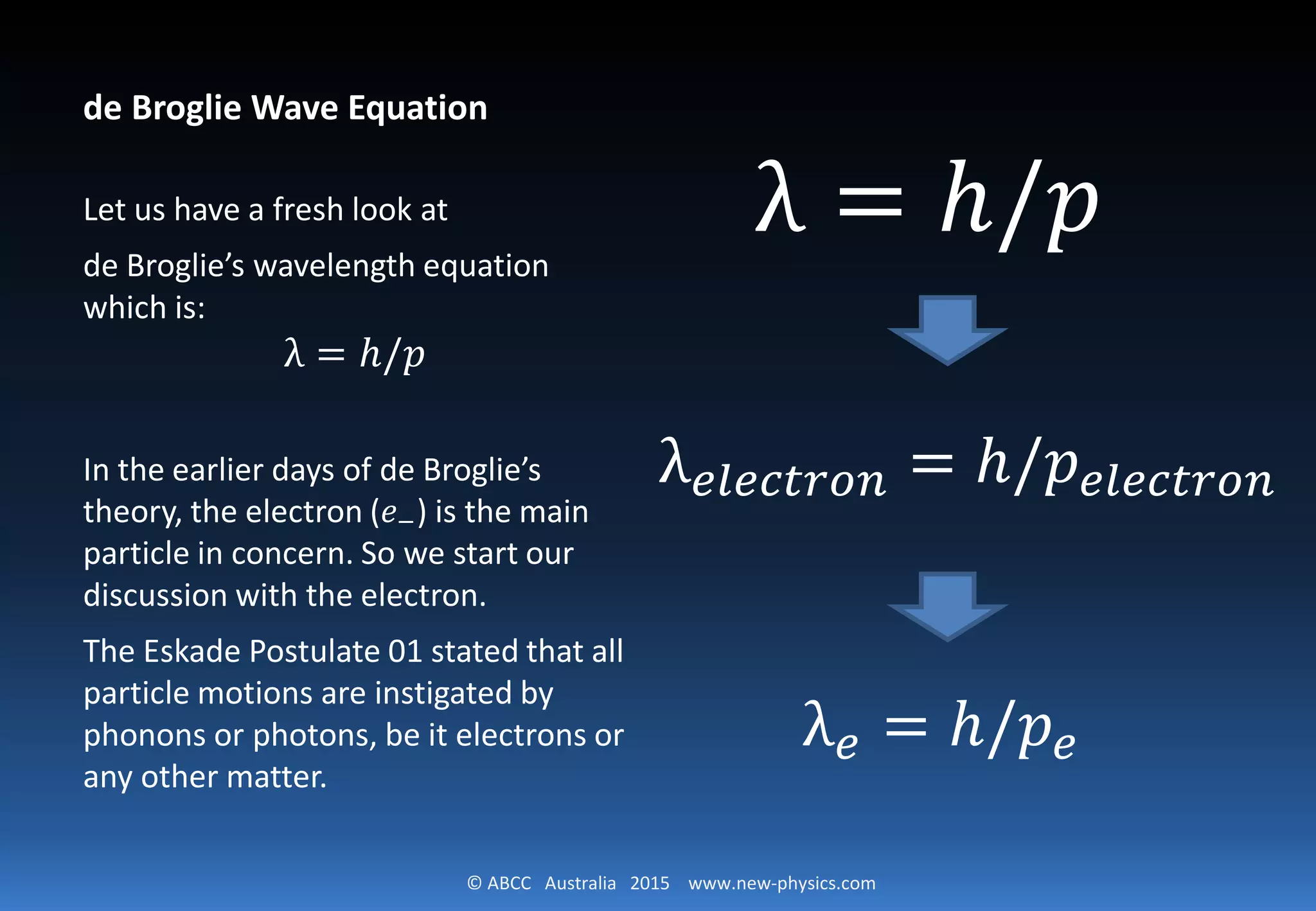
1) The document derives de Broglie's wave equation for the wavelength of an electron from first principles using the carrier postulate that electrons are moved by photons.
2) It shows that the momentum and kinetic energy of the moving electron is equal to the momentum and energy of the incorporated photon.
3) This leads to the derivation of de Broglie's wave equation that the wavelength of the electron is equal to Planck's constant h divided by the momentum of the electron.