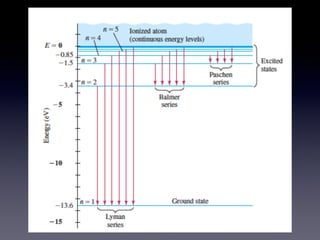
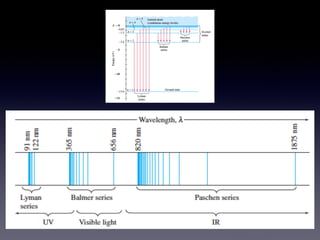
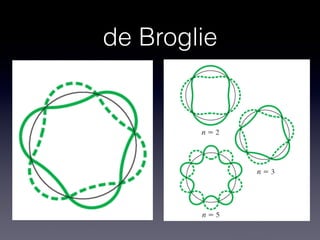
The document discusses key concepts in the development of quantum theory including:
1) De Broglie hypothesized that particles like electrons exhibit wave-like properties and can have an associated wavelength.
2) Schrodinger used de Broglie's idea to develop the first quantum theory based on wave equations to describe electrons rather than classical orbits.
3) Heisenberg formulated the uncertainty principle - that the exact position and momentum of a particle cannot be known simultaneously.
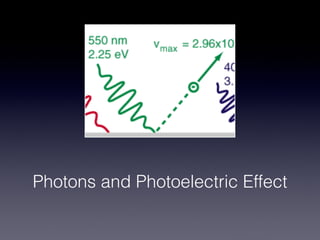
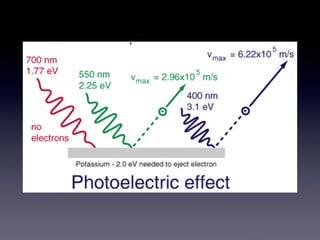
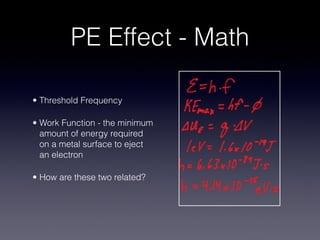
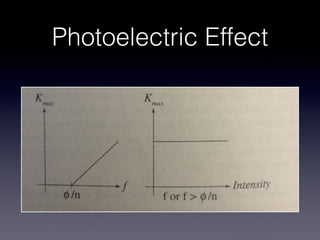
4) Experiments on the photoelectric effect showed photons eject electrons from metals immediately, contradicting classical wave theory but supporting a particle-like nature for photons.