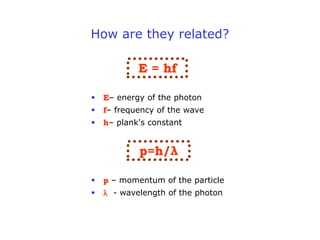
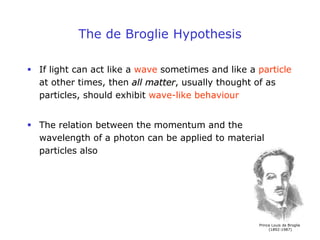
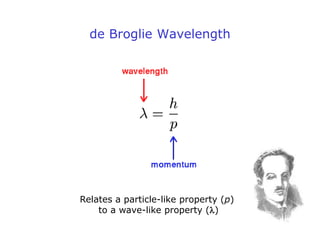
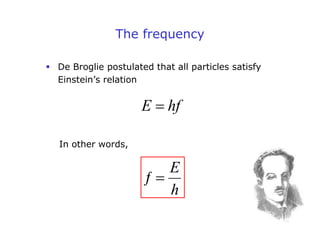
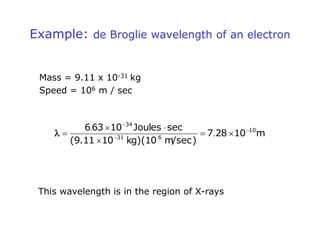
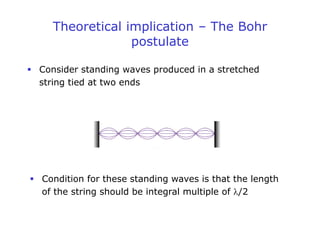
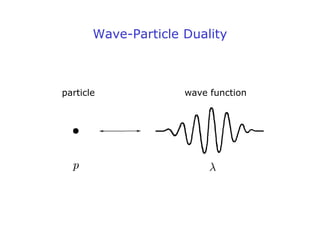
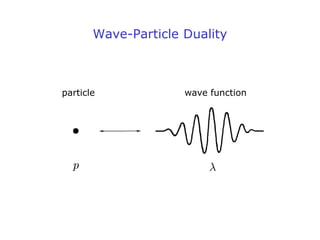
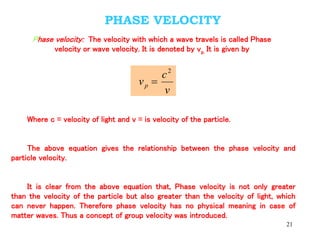
1. Louis de Broglie hypothesized that all matter exhibits both particle-like and wave-like properties, with the wavelength of the matter wave related to the particle's momentum by the de Broglie equation.
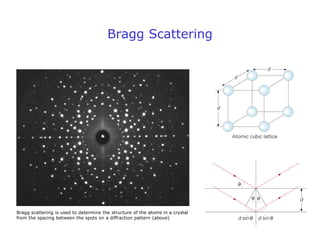
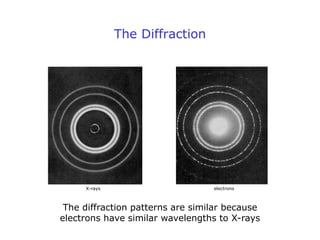
2. Experiments demonstrating the wave-like behavior of electrons and other particles validated de Broglie's hypothesis. Electron diffraction and interference patterns exhibited wave-like characteristics.
3. The wave function describes a particle's quantum mechanical state and contains all observable properties of the particle. It represents the matter wave associated with the particle.