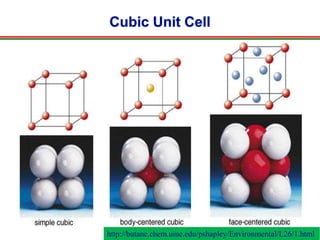
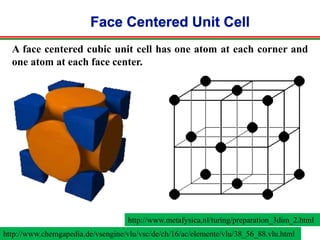
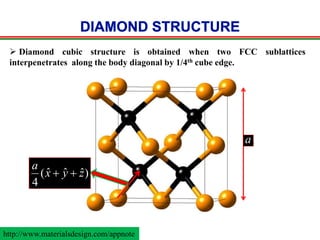
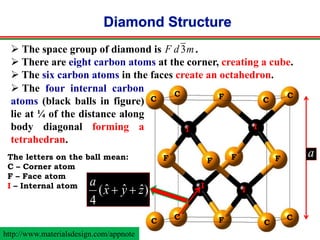
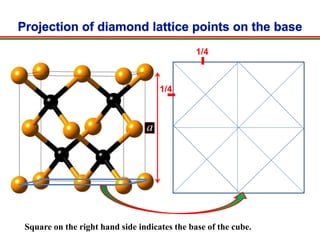
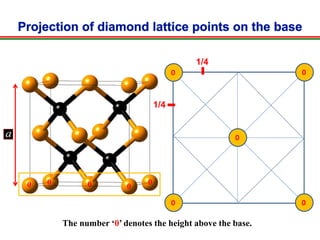
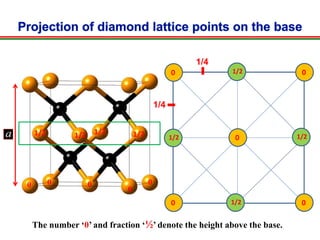
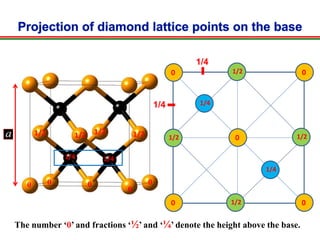
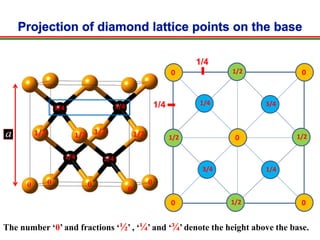
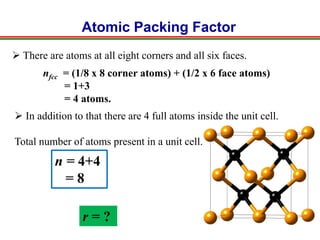
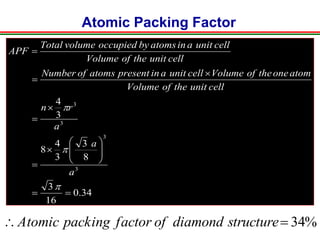
The document discusses the structure of diamond and face-centered cubic (fcc) unit cells, detailing atom positioning, space group, and atomic packing factor calculations. It highlights that the diamond cubic structure is formed by two interpenetrating fcc sublattices and provides formulas to compute the atomic packing factor. Additional resources and contact information are provided for further inquiries.