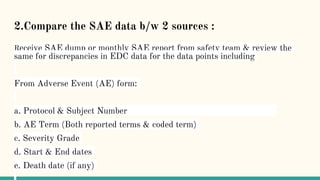
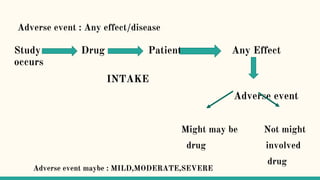
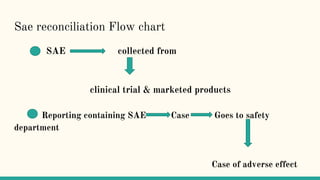
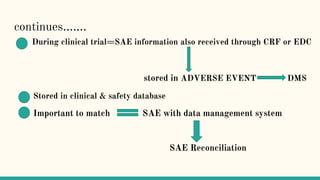
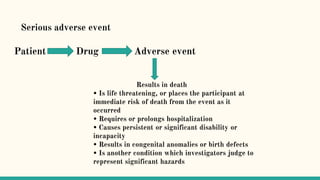
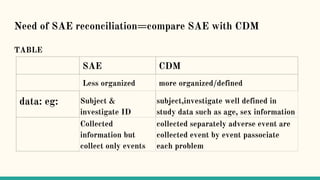
Serious adverse event (SAE) reconciliation is the process of ensuring consistency between clinical and pharmacovigilance databases to maintain patient safety and data integrity in clinical trials. The reconciliation process includes creating a plan, comparing data from both databases to identify discrepancies, and taking necessary corrective actions. Common errors in SAE reconciliation involve data entry mistakes, incomplete records, inconsistent coding or reporting, and delays in reporting.