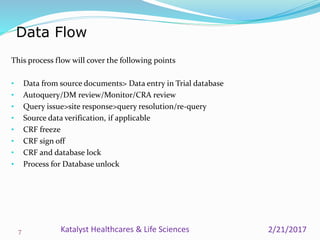
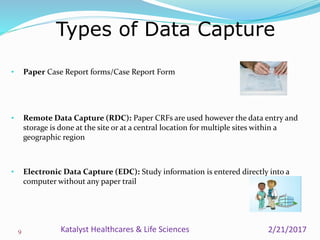
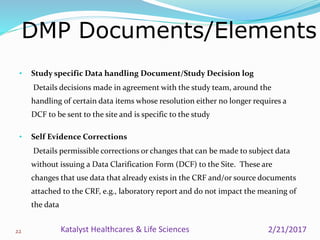
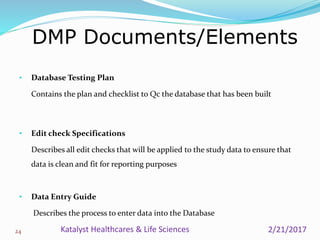
A data management plan (DMP) ensures consistent and effective clinical data management practices throughout a clinical trial. The DMP describes all data management activities, roles, and responsibilities to promote standardized data handling. It provides an agreement between parties on data management deliverables. The DMP covers components like data flow, capture, setup, entry, transfer, processing, coding, safety handling, external data, and database locking. It serves to plan, communicate, and reference data management tasks. Developing a thorough DMP helps ensure quality and regulatory compliance in data collection and analysis.