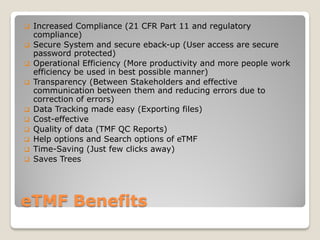
An eTMF is an electronic system for organizing and storing documents related to clinical trials. It replaces paper regulatory binders with electronic storage in a computer server or cloud. Key benefits include increased compliance, efficiency, transparency, and cost savings compared to paper-based systems. While adoption of eTMFs is growing, challenges remain around scope, performance for large document volumes, and change management for training users.







![Current Softwares
eTMF Type Company Clients
Vault eTMF Veena
[Abbott, Alkermes,
Bayer, inVentiv,
Abbvie]
—Trial Interactive TransPerfect
—[Novartis, INC
Research, Infinity,
Sanofi-Aventis, Pfizer,
others]
—
eNNOV eTMF eNNOV -
—
Freyr eTMF Freyr -
SureClinical eTMF SureClinical -](https://image.slidesharecdn.com/etmfppt-151008043438-lva1-app6892/85/eTMF-ppt-10-320.jpg)