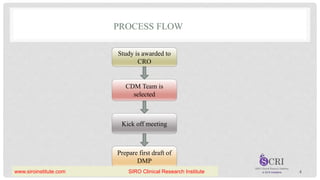
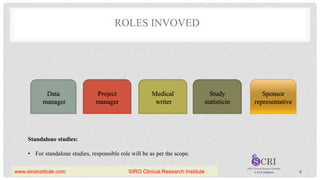
The document outlines the components and significance of a Data Management Plan (DMP) for clinical trials, detailing its purpose, creation process, involved roles, and benefits such as ensuring compliance and quality of data management. It emphasizes that the DMP should be a dynamic document that captures changes throughout the study's lifecycle and must be approved by responsible parties before the study begins. Additionally, the document lists essential components for a DMP, including protocol summary, personnel identification, timelines, and data management processes.