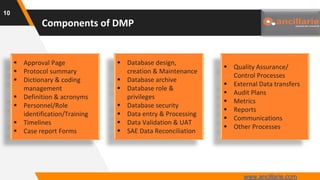
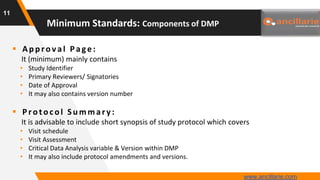
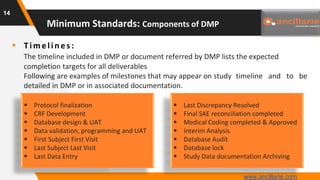
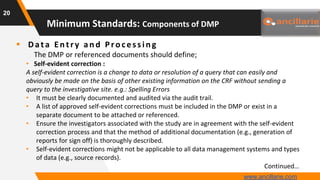
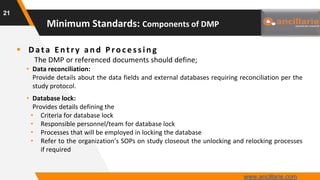
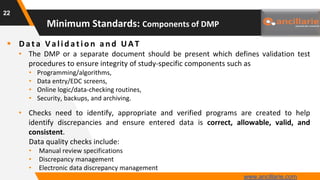
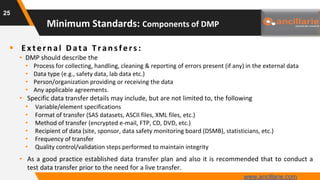
The document provides guidance on key components and best practices for developing a data management plan (DMP). It outlines minimum standards for DMP components, including approval pages, protocol summaries, personnel roles, timelines, case report forms, database design, data validation, quality control processes, auditing, and other key areas. Developing a comprehensive DMP in collaboration with stakeholders helps ensure consistent and compliant data management practices are followed throughout the study lifecycle.