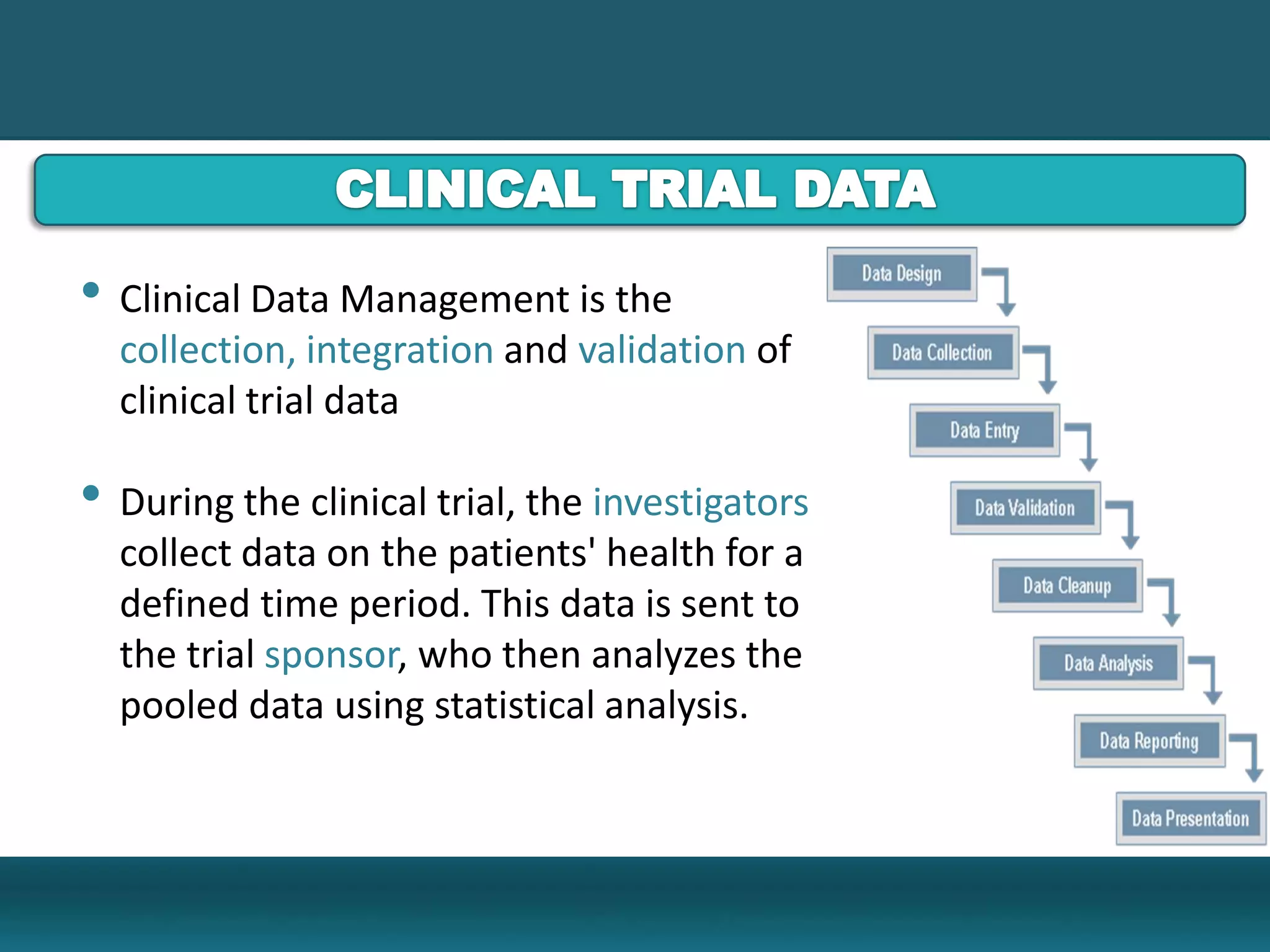
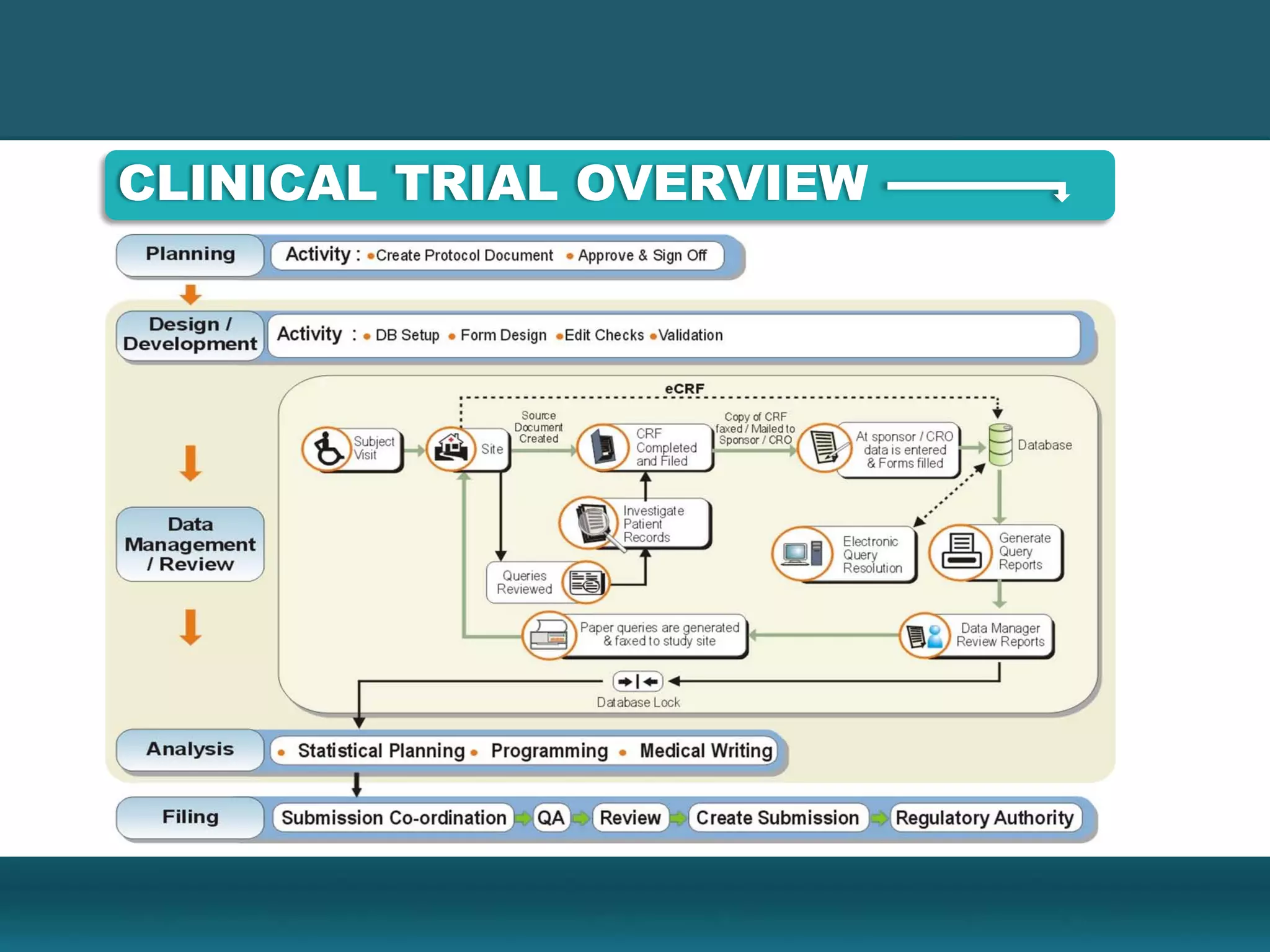
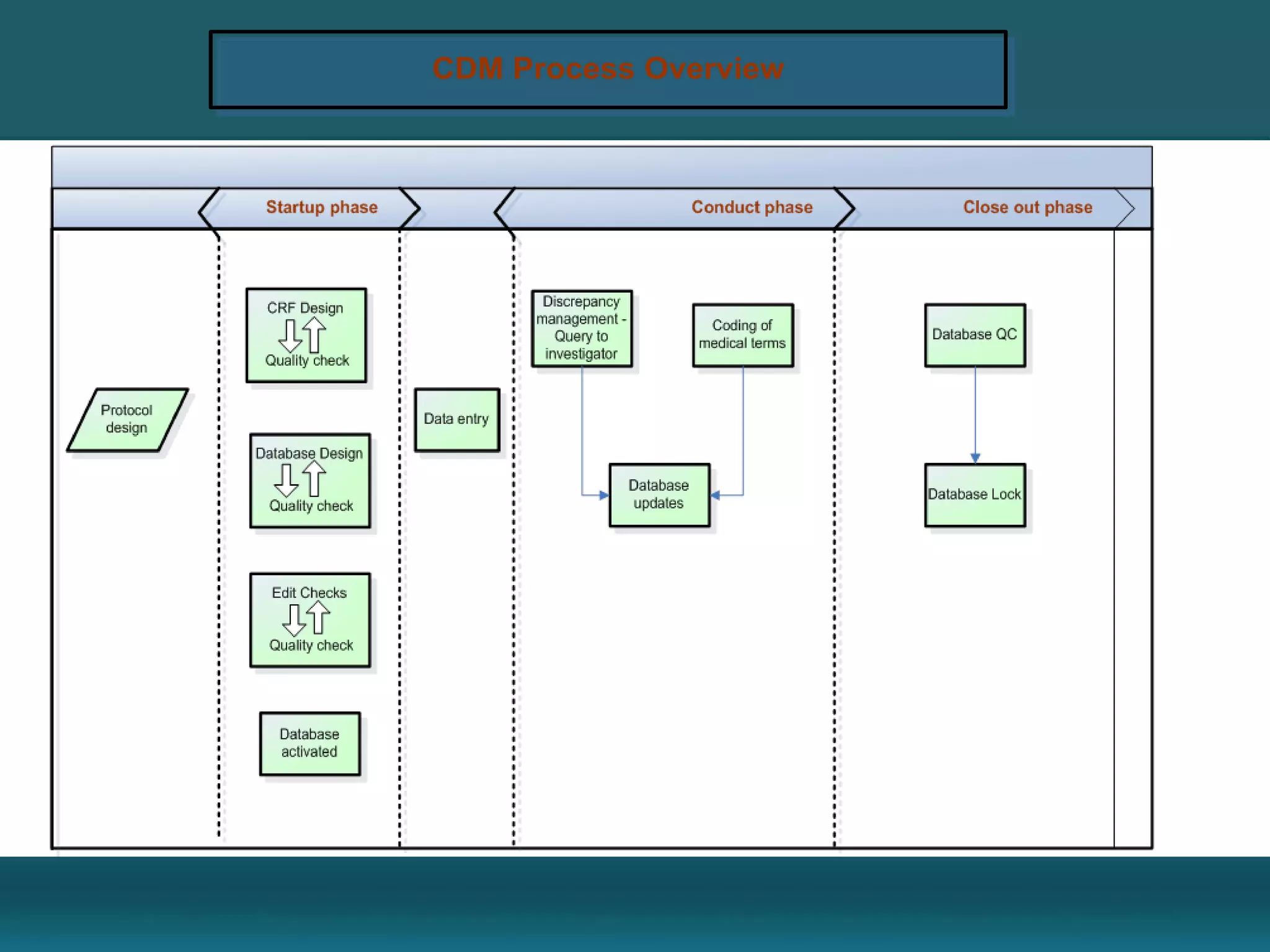
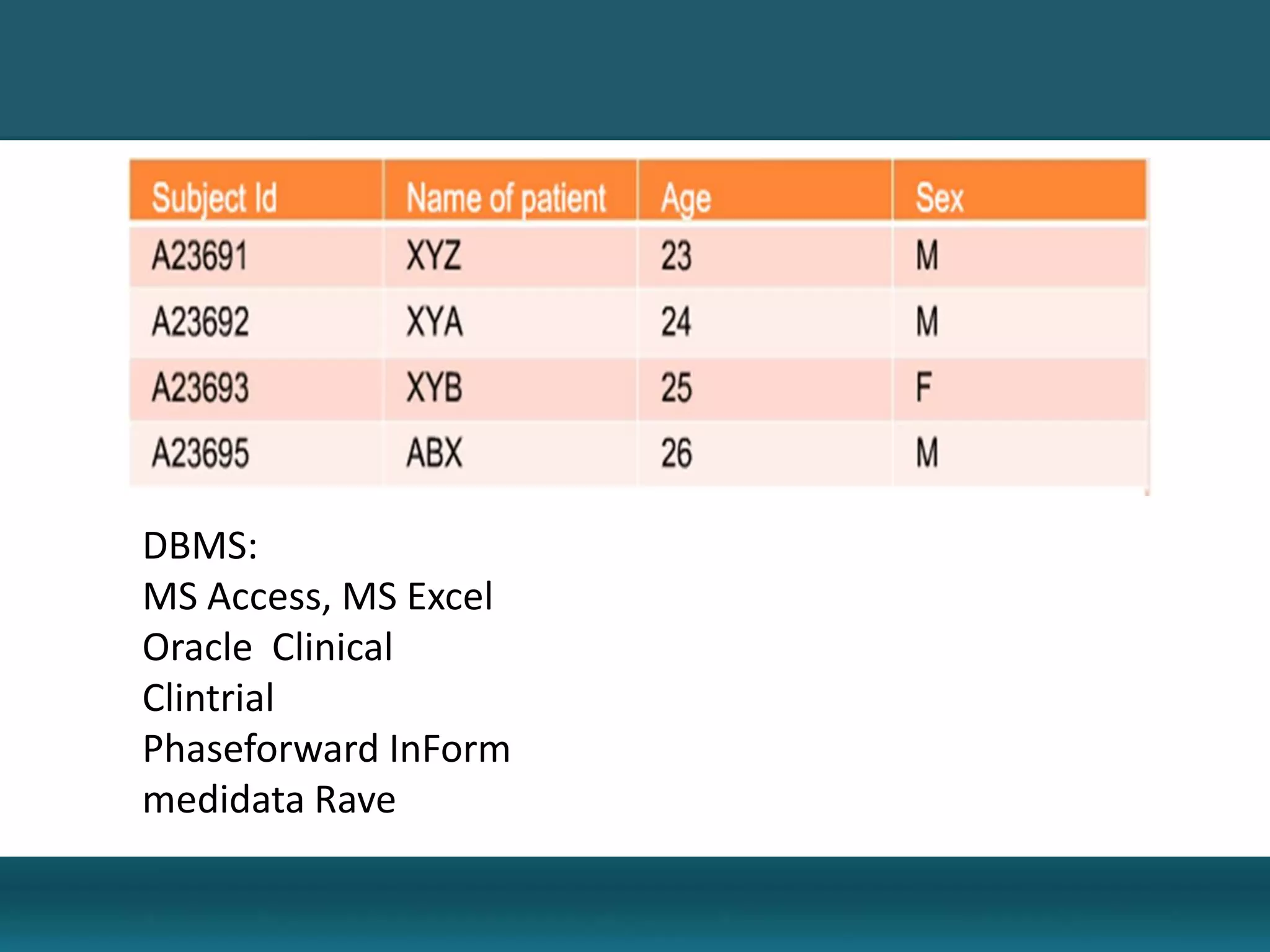
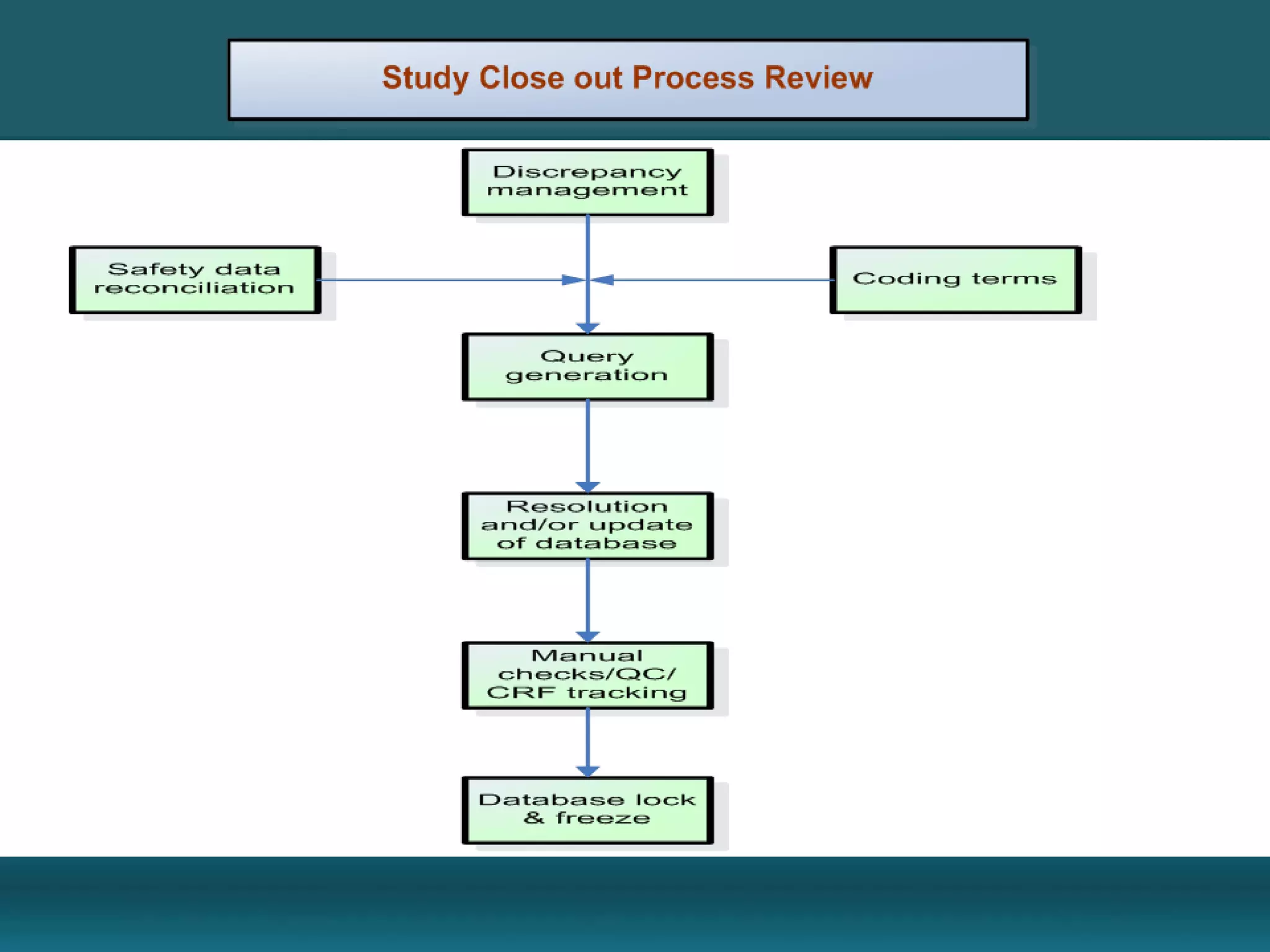
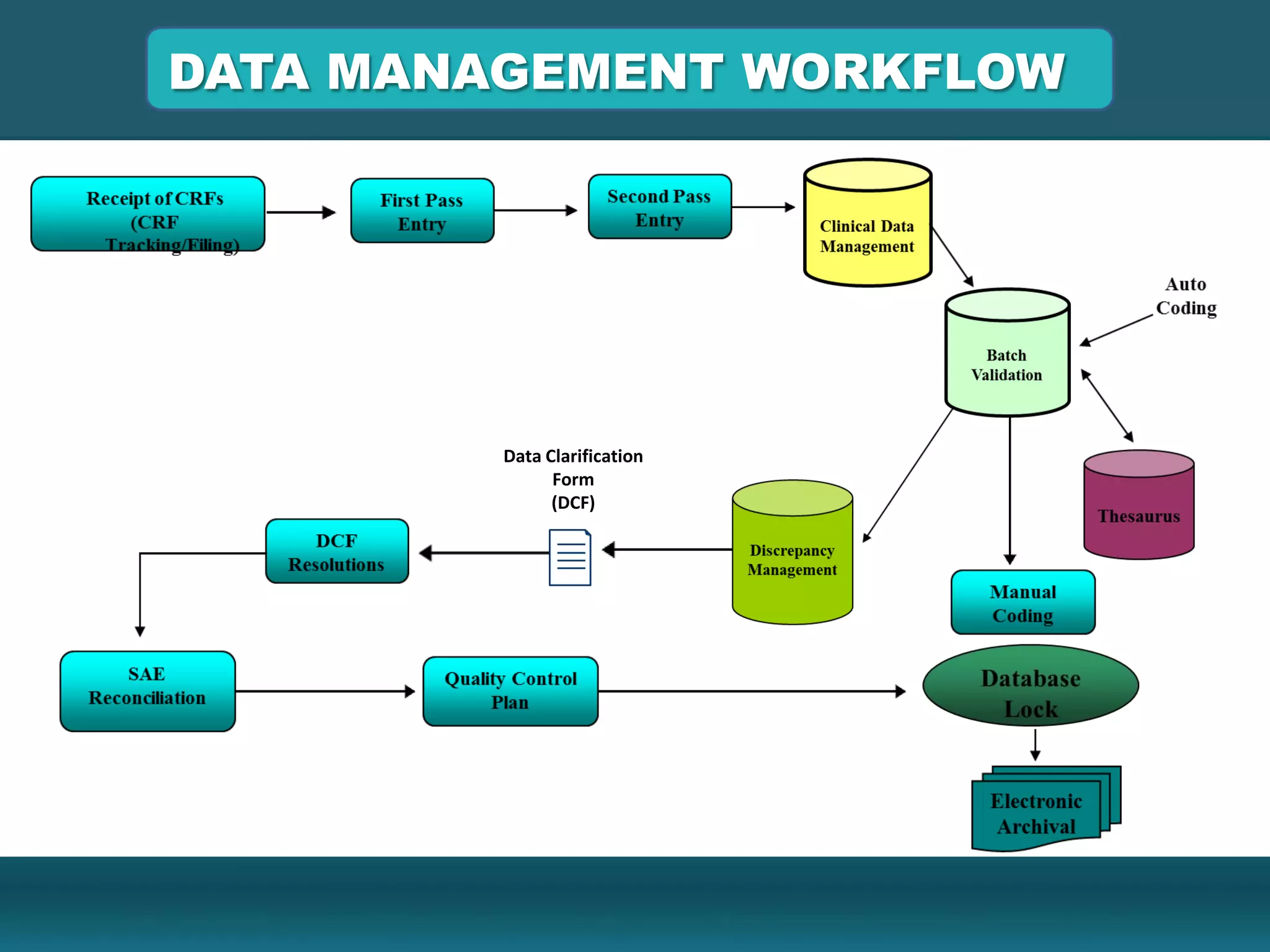
Clinical Data Management (CDM) encompasses the collection, integration, and validation of clinical trial data, ensuring integrity and adherence to quality standards. Key activities include study setup, data entry, discrepancy management, and quality control leading to a database lock, which signifies the data is ready for statistical analysis. CDM is crucial for regulatory approval, ensuring that data from clinical trials is accurate and reliable.