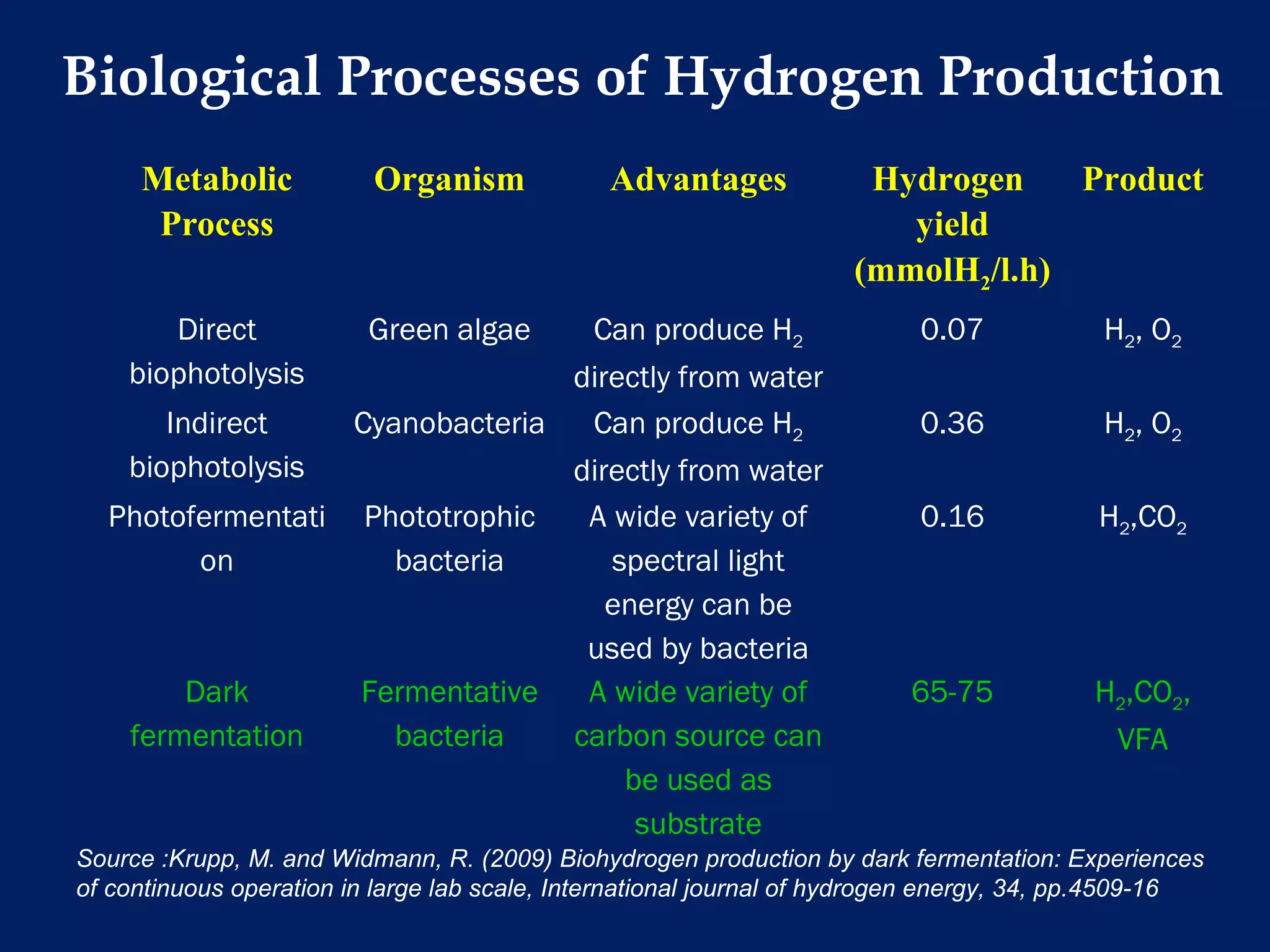
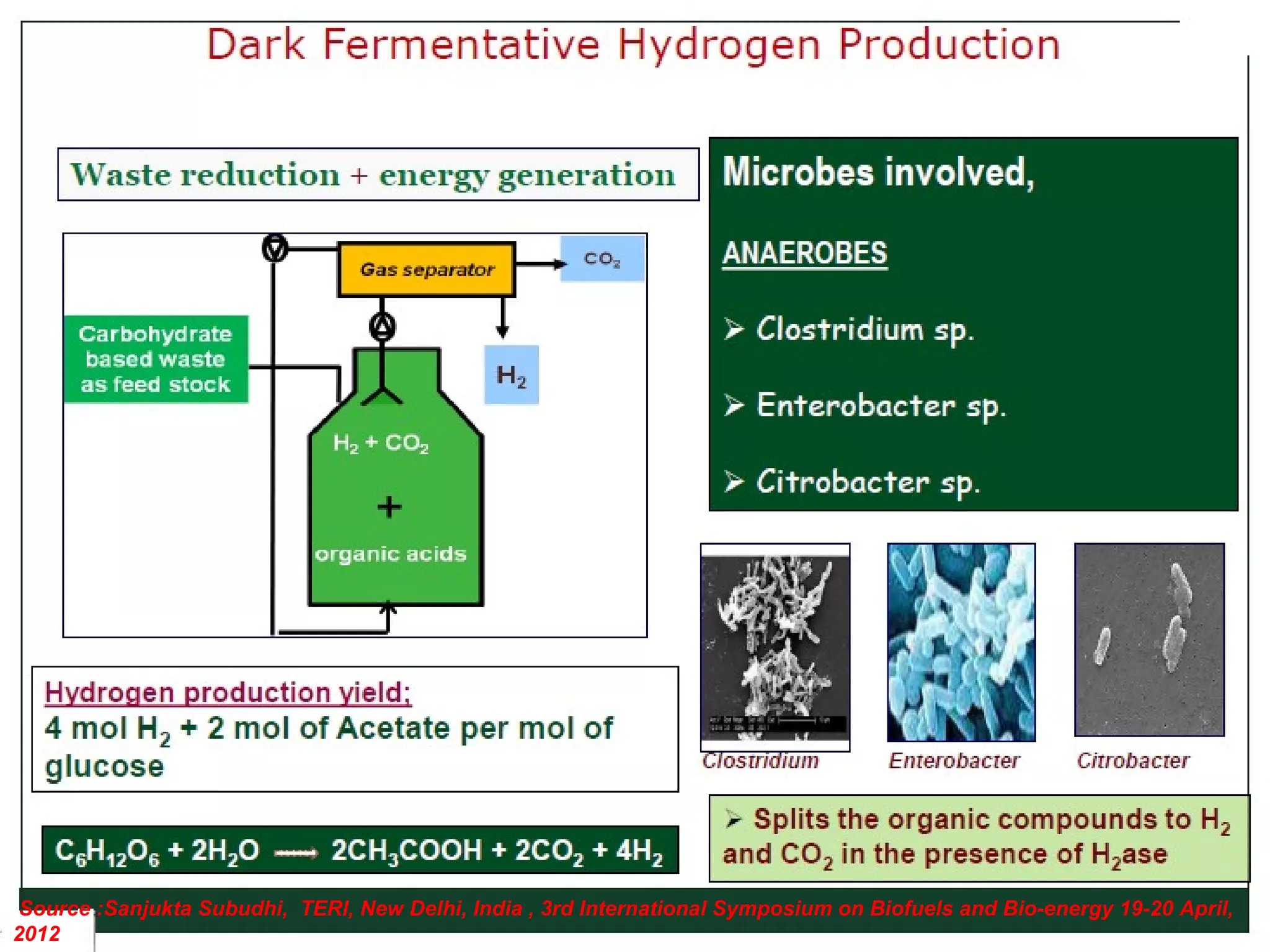
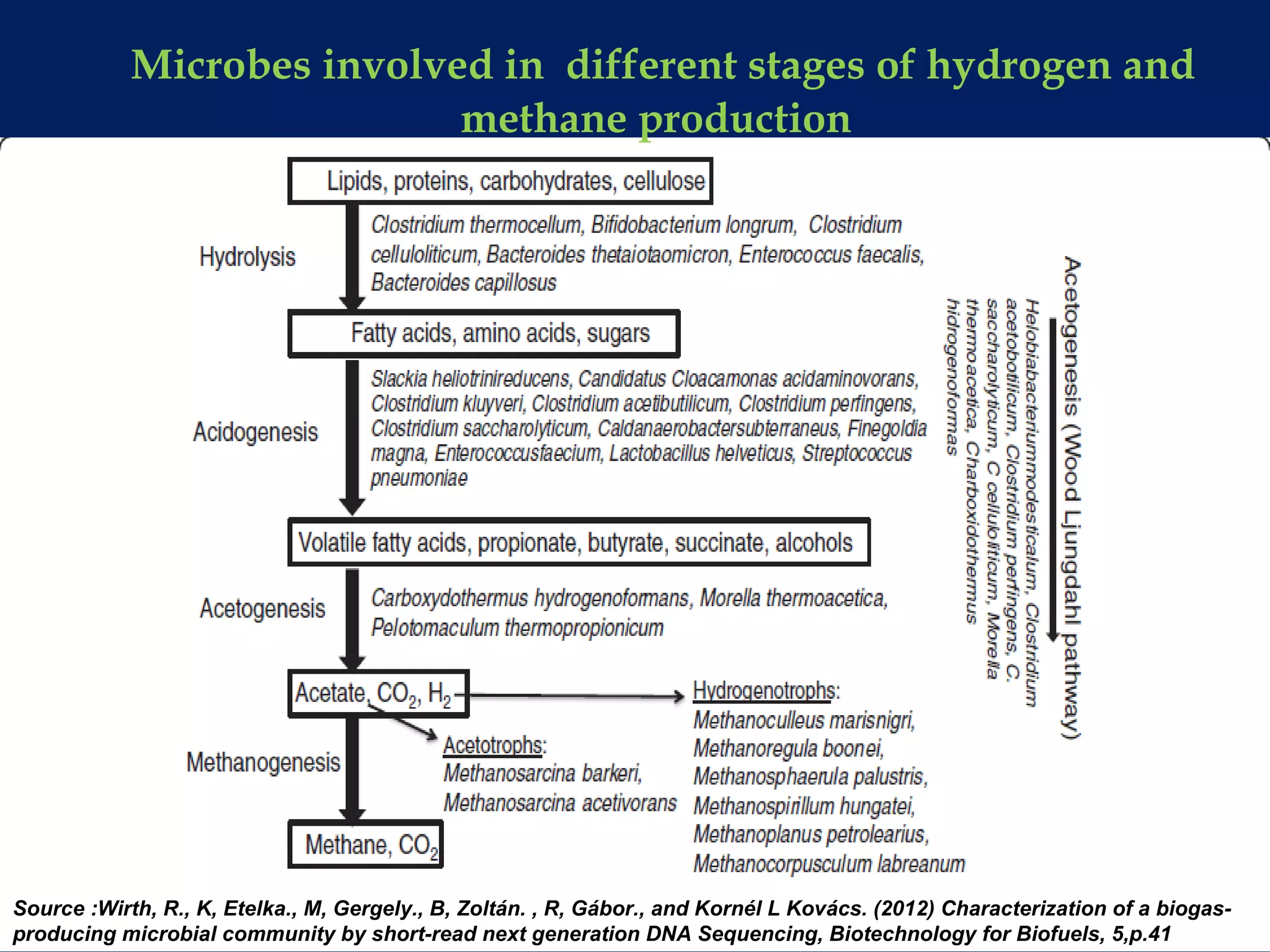
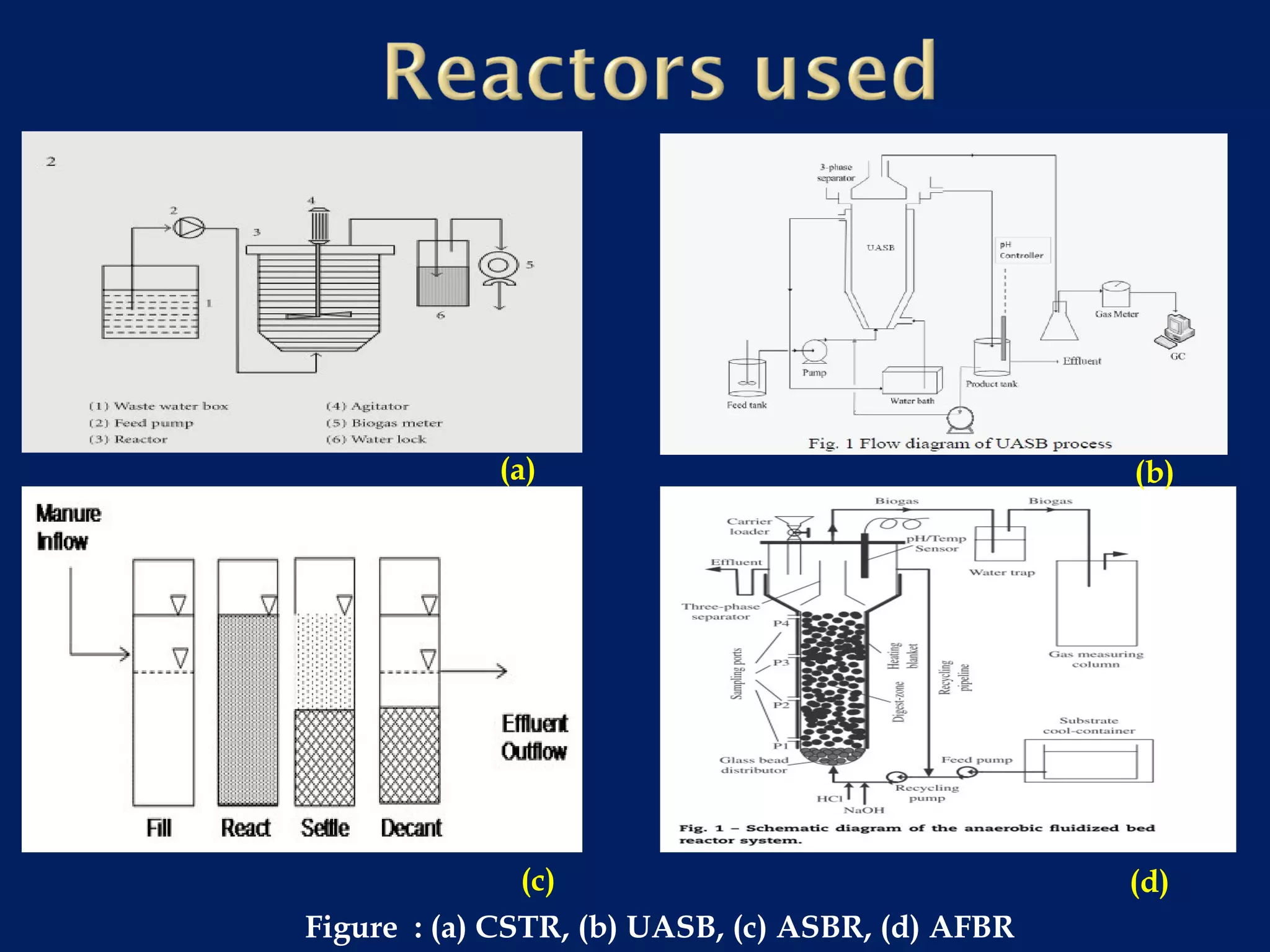
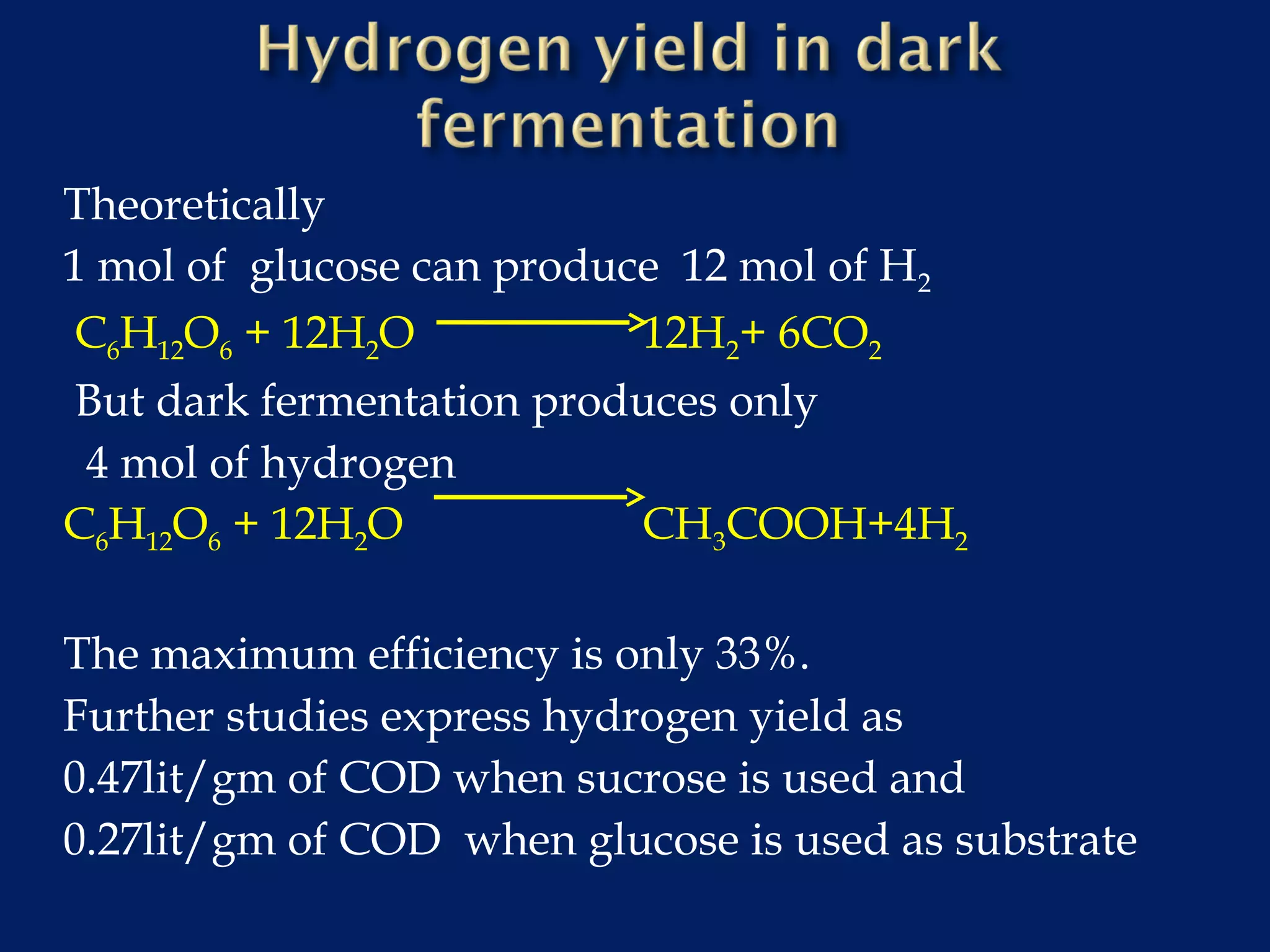
The document discusses hydrogen production from renewable sources, highlighting its advantages such as zero greenhouse gas emissions and potential as an automotive fuel. It outlines various biological processes, particularly dark fermentation and the microbial communities involved, along with substrate types used for hydrogen production. The integration of hydrogen and methane production offers eco-friendly waste reduction and the generation of valuable metabolites.