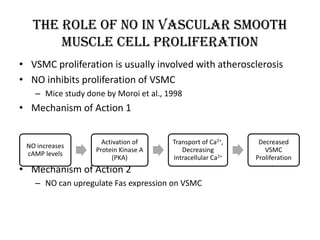
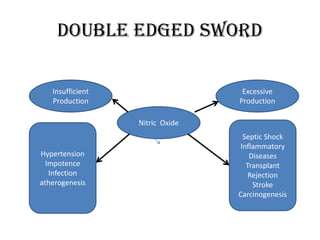
Nitric oxide (NO) is a gas that acts as a signaling molecule in various physiological processes. It is produced by nitric oxide synthase enzymes from arginine and oxygen. NO signals the dilation of blood vessels by diffusing into endothelial cells and increasing cGMP levels, which leads to smooth muscle relaxation. It also prevents platelet aggregation, acts as a neurotransmitter, and is involved in the immune response by assisting macrophages in killing bacteria. While NO is important for many functions, too much or too little production can be harmful and lead to conditions like hypertension or infection.