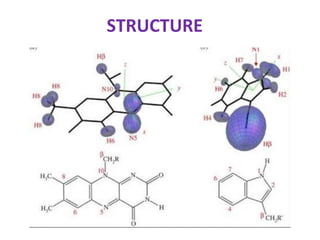
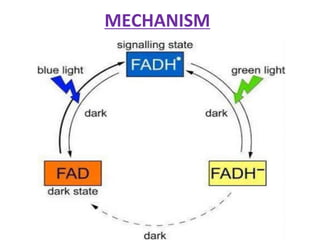
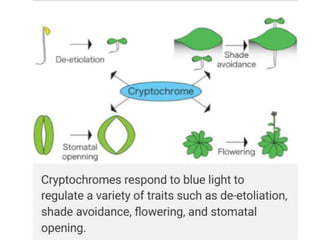
Cryptochrome is a class of flavoprotein that acts as a blue light photoreceptor involved in circadian rhythms in plants and animals. It was first observed in plants in the 1800s but was not identified until the 1980s. Cryptochrome has a similar structure to photolyase but serves different functions. It contains a single molecule of FAD that absorbs blue light. In plants, cryptochrome mediates responses to blue light such as growth, seedling development, and leaf/stem expansion.