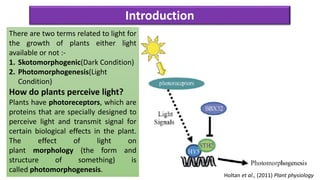
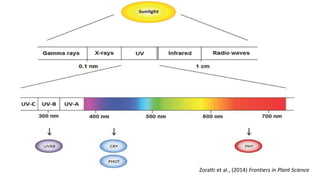
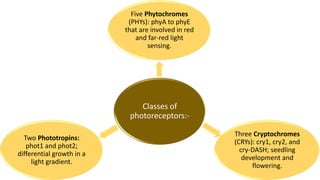
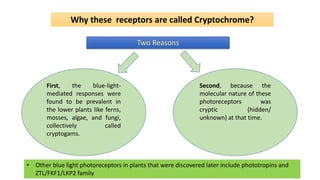
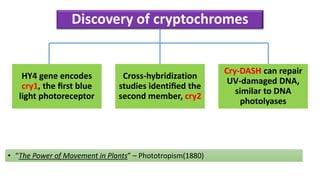
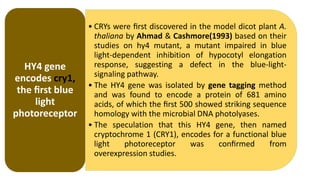
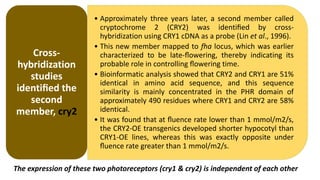
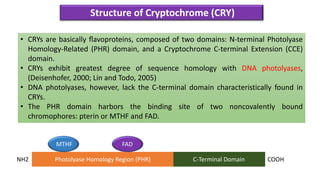
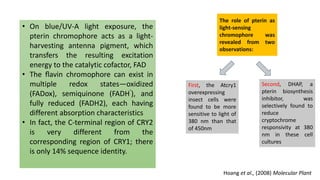
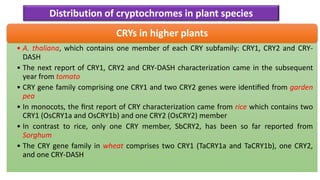
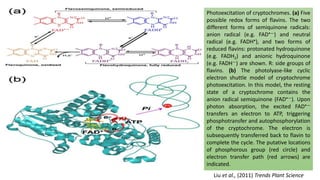
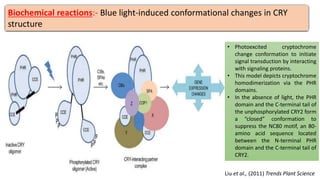
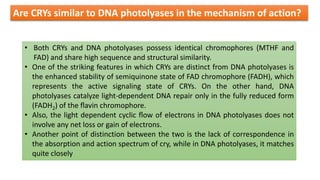
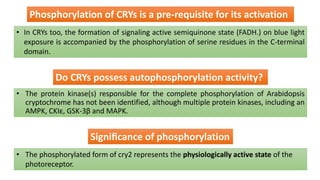
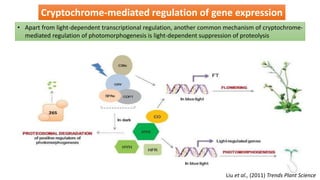
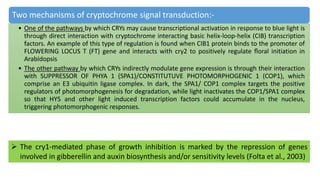
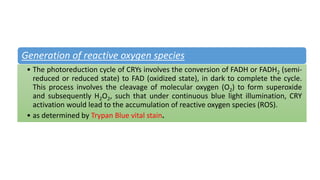
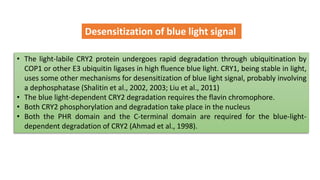
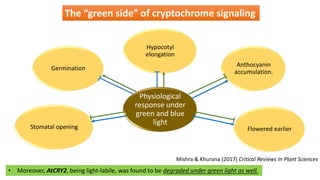
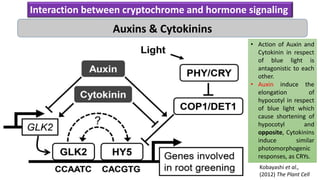
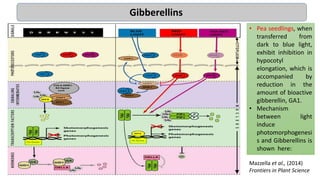
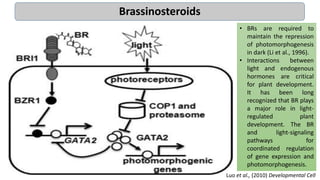
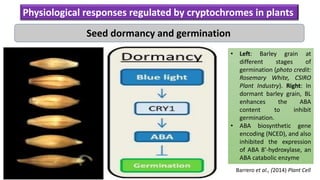
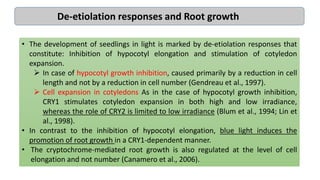
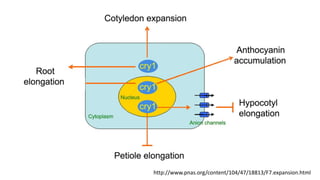
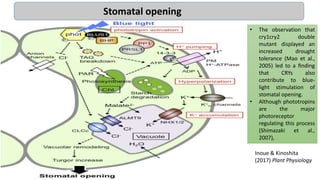
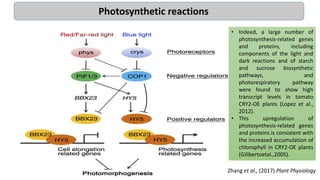
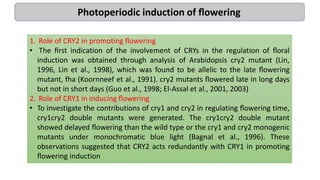
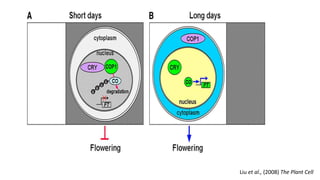
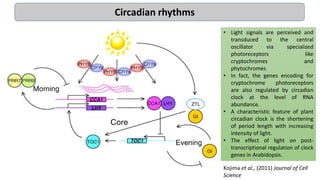
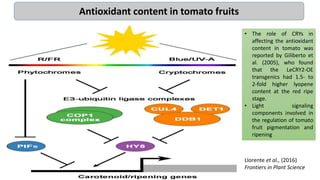
Cryptochromes are blue light photoreceptors in plants that regulate important physiological processes. They were first discovered in Arabidopsis thaliana and are structurally similar to microbial DNA photolyases, containing a photolyase homology region and C-terminal extension. Cryptochromes exist in multiple redox states of their flavin chromophore, and blue light excitation triggers a conformational change involved in signaling. Cryptochromes interact with hormone signaling pathways to control processes like seedling development and flowering time.