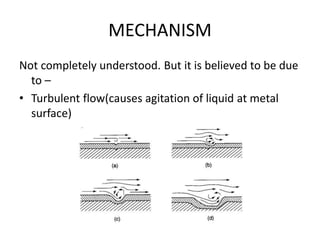
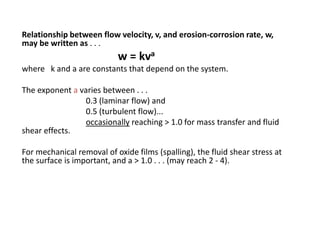
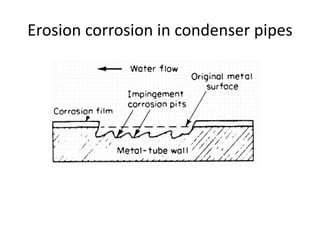
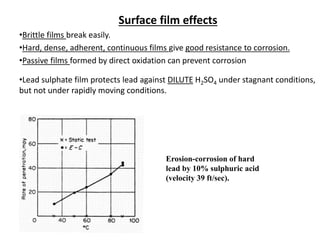
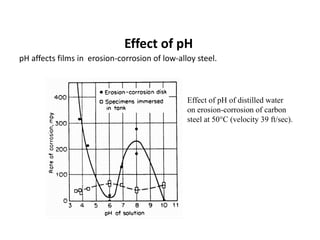
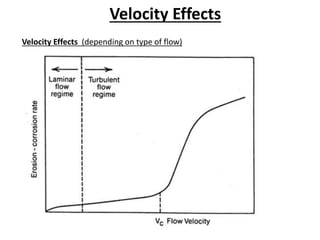
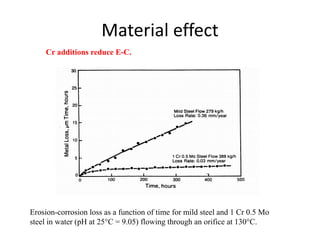
Rishabh Sharma's presentation discusses erosion corrosion, which is an increase in corrosion caused by a high relative velocity between a corrosive environment and a metal surface. It involves both chemical corrosion and mechanical wear as corroded metal is removed. The mechanisms are not fully understood but involve turbulent flow, suspended solids, and gas/liquid interactions. Erosion corrosion is more severe for softer metals and in equipment exposed to high velocities, turbulence, and mass transfer. Examples include pipes, valves, pumps and turbine blades. The presentation covers factors like pH, velocity, material choice, and surface films that influence erosion corrosion rates and provides prevention methods like design changes, environment modifications, material selection, and coatings.